Zenarestat
- CAS NO.:112733-06-9
- Empirical Formula: C17H11BrClFN2O4
- Molecular Weight: 441.639
- MDL number: MFCD00865809
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:10:42
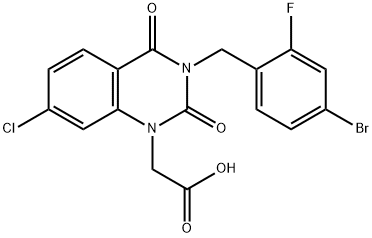
What is Zenarestat?
Originator
FK 366 ,Fujisawa Pharmaceutical
The Uses of Zenarestat
Treatment of diabetic neuropathy (aldose reductase inhibitor).
Manufacturing Process
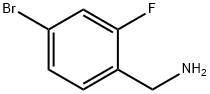
To a solution of 4-bromo-2-fluorobenzylamine and triethylamine in chloroform
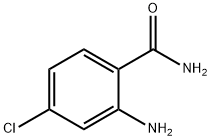
was added dropwise a solution of 4-chloro-2-nitrobenzoyl chloride in
chloroform at 0°C with stirring and the mixture was stirred at the same
temperature for 1 h. The reaction mixture was washed in turn with diluted
aqueous hydrochloric acid and water, and then dried. Evaporation of the
solvent followed by recrystallization from diethyl ether gave N-(4-bromo-2-
fluorobenzyl)-4-chloro-2-nitrobenzamide.
A mixture of N-(4-bromo-2-fluorobenzyl)-4-chloro-2-nitrobenzamide and iron
(1.45 g) in acetic acid (66 ml) was stirred at 100°C for 30 min. After cooling,
iron was filtered off. The filtrate was evaporated to give a residue, which was
made alkaline with aqueous 1 N sodium hydroxide and extracted with ethyl
acetate. The extract was washed with water and dried. Removal of the solvent
gave 2-amino-N-(4-bromo-2-fluorobenzyl)-4-chlorobenzamide.
2-Amino-N-(4-bromo-2-fluorobenzyl)-4-chlorobenzamide and N,N'-
carbonyldiimidazole were dissolved in dioxane (50 ml). The solution was
evaporated to give a residue, which was stirred at 150°C for 30 min. After
cooling, the precipitates were collected by filtration and washed with ethanol
to give 3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-2,4-
dioxoquinazoline; melting point >280°C.
To a suspension of 3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-
2,4-dioxoquinazoline in N,N-dimethylformamide was added sodium hydride
(60% in mineral oil) with stirring at 0°C and the mixture was stirred for 15
min at the same temperature. To this mixture was added ethyl bromoacetate
and the mixture was stirred for 1 h at room temperature. The reaction
mixture was poured into diluted hydrochloric acid and extracted with ethyl
acetate. The extract was washed with brine, dried and evaporated to give a
residue. Thus obtained product was purified by recrystallization from isopropyl
ether to give 2-[3-(4-bromo-2-fluorobenzyl)-7-chloro-1,2,3,4-tetrahydro-2,4-
dioxoquinazolin-1-yl]acetic acid melting point 223°-224°C.
Therapeutic Function
Aldose reductase inhibitor
Properties of Zenarestat
| Melting point: | 223-224° |
| Boiling point: | 624.4±65.0 °C(Predicted) |
| Density | 1.737 |
| solubility | DMSO : 100 mg/mL (226.43 mM; Need ultrasonic) |
| pka | 3.76±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Zenarestat
Computed Descriptors for Zenarestat
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![2-[(3-chlorophenyl)amino]acetic acid](https://img.chemicalbook.in/CAS/GIF/10242-05-4.gif)
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
