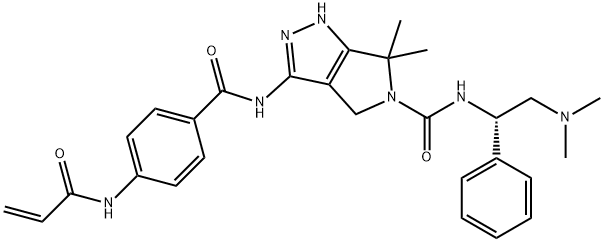
YKL-5-124
Synonym(s):(S)-3-(4-acrylamidobenzamido)-N-(2-(dimethylamino)-1-phenylethyl)-6,6-dimethyl-4,6-dihydropyrrolo[3,4-c]pyrazole-5(1H)-carboxamide;N-[(1S)-2-(Dimethylamino)-1-phenylethyl]-4,6-dihydro-6,6-dimethyl-3-[[4-[(1-oxo-2-propen-1-yl)amino]benzoyl]amino]-pyrrolo[3,4-c]pyrazole-5(1H)-carboxamide
- CAS NO.:1957203-01-8
- Empirical Formula: C28H33N7O3
- Molecular Weight: 515.61
- MDL number: MFCD34469131
- Update Date: 2026-01-29 15:22:00
What is YKL-5-124?
Biological Activity
YKL-5-124 is a potent, selective, irreversible and covalent CDK7 inhibitor with IC50s of 53.5 nM and 9.7 nM for CDK7 and CDK7/Mat1/CycH, respectively. YKL-5-124 is >100-fold greater selective for CDK7 than CDK9 and CDK2, and inactive against CDK12 and CDK13. YKL-5-124 induces a strong cell-cycle arrest, inhibits E2F-driven gene expression, and exhibits little effect on RNA polymerase II phosphorylation status[1]. YKL-5-124 (0-2000 nM; 72 hours; HAP1 cells) treatment causes a dose-dependent increase in G1- and G2/M-phase cells and a corresponding loss of S-phase cells[1].YKL-5-124 (0-2000 nM; 24 hours; HAP1 WT cells) treatment inhibits CDK1 T-loop phosphorylation, and to a lesser extent CDK2 T-loop phosphorylation in a concentration-dependent fashion[1].Treatment of cells with YKL-5-124 as a competitor at a concentration of about 30 nM blocks pull-down of CDK7-cyclin H but has no effect on the pull-down of cyclin K-CDK12/13 in HAP1 cells. Treatment with 100 nM YKL-5-124 reduces CDK7-cyclin H binding to bioTHZ1 by >50% at 30 min[1].
Storage
Store at -20°C
References
[1]. Olson CM, et al. Development of a Selective CDK7 Covalent Inhibitor Reveals Predominant Cell-Cycle Phenotype. Cell Chem Biol. 2019 Jun 20;26(6):792-803.e10.
Properties of YKL-5-124
| Boiling point: | 706.1±60.0 °C(Predicted) |
| Density | 1.284±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : 250 mg/mL (484.86 mM; Need ultrasonic) |
| pka | 12.78±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for YKL-5-124
Computed Descriptors for YKL-5-124
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 YKL-5-124 CAS 1957203-01-8View Details
YKL-5-124 CAS 1957203-01-8View Details
1957203-01-8 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5