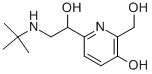
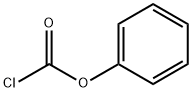
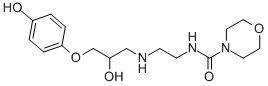
XAMOTEROL HEMIFUMARATE
- CAS NO.:81801-12-9
- Empirical Formula: C16H25N3O5.C4H4O4
- Molecular Weight: 455.461
- MDL number: MFCD00661103
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 11:31:08
What is XAMOTEROL HEMIFUMARATE?
Originator
Sepan,Yamanouchi
The Uses of XAMOTEROL HEMIFUMARATE
Stimulant (cardiac).
The Uses of XAMOTEROL HEMIFUMARATE
Xamoterol is an authentic β1-adrenoceptor (β1-AR) agonist that has been shown to mimic the autoantibody effect on rat atria β1-AR apoptosis.
Background
Xamoterol is a β1-adrenoceptor partial agonist that has shown to improve systolic and diastolic function in studies with heart failure patients. It modulates the sympathetic control of the heart but has no agonist action on β2-adrenoceptors.
Definition
ChEBI: Xamoterol is a member of morpholines.
Manufacturing Process
A suspension of 1-p-benzyloxyphenoxy-2,3-epoxypropane (11.5 g) in
isopropanol (6 ml) is added to a stirred mixture of 4-(N-beta-
aminoethylcarbamoyl) morpholine hydrogen sulphate (12.7 g), potassium
hydroxide (7.0 g) and isopropanol (10 ml) and the mixture is stirred at 45°C
for 1 hour and then evaporated to dryness under reduced pressure. The
residual oil is stirred with water, the mixture is filtered and the solid residue is
dissolved in acetone. A 30% solution of hydrogen chloride in propanol is
added until the pH of the mixture is less than 2, and the mixture is filtered.
The solid residue is crystallised from water and there is thus obtained 1-p-
benzyloxyphenoxy-3-(beta-morpholinocarbonamidoethyl)amino-2-propanol
hydrochloride (4.9 g).
A solution of the above compound in a mixture of ethanol (20 ml) and acetic
acid (20 ml) is shaken with a 30% palladium-on-charcoal catalyst (0.1 g) in
an atmosphere of hydrogen at laboratory temperature and pressure until 250
ml of hydrogen is absorbed. The mixture is filtered, the filtrate is evaporated
to dryness under reduced pressure and to the residue is added a hot solution
of fumaric acid (1.25 g) in ethanol (15 ml). The mixture is kept at 5°C for 12
hours and is then filtered, and the solid residue is washed with hot ethanol
and then dried. There is thus obtained 1-p-hydroxyphenoxy-3-beta-
(morpholinocarbonamido)ethyl-amino-2-propanol hydrogen fumarate, m.p.
168-169°C (with decomposition).
The 4-(N-beta-aminoethylcarbamoyl)morpholine hydrogen sulphate used as
starting material may be obtained as follows:
Morpholine (4.35 g) and phenyl chloroformate (6.35 g) are separately and
simultaneously added dropwise during 20 min to a stirred mixture of toluene
(10 ml), water (5 ml) and sodium hydroxide (2 g) which is maintained at 0°C.
The mixture is stirred for a further 2 hours whilst the temperature is allowed
to rise to 20°C. The toluene solution is separated, the aqueous solution is extracted twice with toluene and the combined toluene solutions are washed
with water, dried and evaporated to dryness under reduced pressure. The
residue is crystallised from petroleum ether (boiling point 60-80°C) and there
is thus obtained N-phenoxycarbonylmorpholine, melting point 46.5-47.5°C.
A mixture of the above compound (11 g) and ethylenediamine (27.8 g) is
stirred at laboratory temperature for 3 days and the excess of ethylene
diamine is removed by evaporation under reduced pressure. The residue is
dissolved in methanol, the solution is cooled to 5°C and concentrated sulfuric
acid is added until the pH of the solution is 2. A filter-aid (Celite, 10 g) is
added and the mixture is stirred for 1 hour and then filtered. The filtrate is
evaporated to dryness under reduced pressure and the residue is stirred with
ethyl acetate. The mixture is filtered and there is thus obtained as solid
residue 4-(N-beta-aminoethylcarbamoyl)morpholine hydrogen sulphate,
melting point 168-169°C.
Therapeutic Function
Beta-adrenergic blocker, Cardiac stimulant
Metabolism
Not Available
Properties of XAMOTEROL HEMIFUMARATE
| Melting point: | 168-170°C |
| solubility | H2O: 10 mg/mL at 60 °C, soluble |
| form | solid |
| color | white |
Safety information for XAMOTEROL HEMIFUMARATE
Computed Descriptors for XAMOTEROL HEMIFUMARATE
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5