Voxelotor
- CAS NO.:1446321-46-5
- Empirical Formula: C19H19N3O3
- Molecular Weight: 337.37
- MDL number: MFCD29053764
- Update Date: 2026-02-02 18:10:39
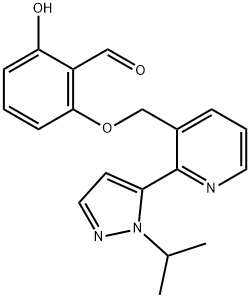
What is Voxelotor?
Absorption
Voxelotor is rapidly absorbed after oral administration, with a plasma Tmax of 2 hours. Tmax in the red blood cells ranges from 17-24 hours. The Cmax in whole blood and red blood cells occur 6 and 18 hours after an oral dose, respectively. Consumption of a high-fat meal with voxelotor significantly increased exposure to the drug during clinical trials. After a daily dose of either 300, 600, or 900 mg for a period of 15 days, when steady-state concentrations were reached, the average RBC Cmax for the respective doses were measured to be 4950, 9610 and 14 000 μg*h mL?1, respectively.
Toxicity
The LD50 information and overdose information is unavailable at this time. Current clinical study results suggest that dose-limiting toxicities of voxelotor are unlikely.
The Uses of Voxelotor
GBT 440 is a potent allosteric effector of sickle cell hemoglobin (Hb) that increases the affinity of Hb for oxygen and inhibits its polymerization when subjected to hypoxic conditions.
Indications
In the US, voxelotor is indicated to treat sickle cell disease in both adult and pediatric patients aged 4 years and older. In Europe, it is indicated for the treatment of hemolytic anemia due to sickle cell disease (SCD) in adults and pediatric patients 12 years of age and older as monotherapy or in combination with hydroxyurea.
Background
Voxelotor is a novel hemoglobin S polymerization inhibitor for the treatment of sickle cell disease. This is a genetically inherited condition most prevalent in the Middle East, Africa, and certain parts of India. Sickle cell disease can lead to excruciating pain, stroke, infection, and various other complications arising from the blockage of blood vessels.
Voxelotor was granted accelerated FDA approval on November 25 2019, as it is likely to be a promising treatment for the 100,000 individuals in the U.S. suffering from the disease, in addition to 20 million others worldwide. It was developed by Global Blood Therapeutics, Inc. and is unique from other drugs used to treat sickle cell anemia, such as hydroxyurea, L-glutamine, and crizanlizumab due to its novel mechanism of action. The EMA approved the use of voxelotor for the treatment of hemolytic anemia associated with sickle cell disease in February 2022.
Pharmacokinetics
Voxelotor increases hemoglobin (Hb) oxygen affinity in a dose-dependent manner. It has led to up to a 40% increase in hemoglobin in clinical trials. Voxelotor may inhibit red blood cell sickling, attenuate red blood cell deformability, and reduce whole blood viscosity.
Metabolism
Voxeletor is heavily metabolized via two phases. Phase I metabolism consists of oxidation and reduction, while phase II metabolism consists of glucuronidation. Voseletor is oxidized mainly by CYP3A4 and by CYP2C19, CYP2B6, and CYP2C9, to a lesser extent.
Properties of Voxelotor
| Melting point: | 95 - 97oC |
| Boiling point: | 539.2±50.0 °C(Predicted) |
| Density | 1.23±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| pka | 7.67±0.10(Predicted) |
| form | Solid |
| color | White to Off-White |
Safety information for Voxelotor
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Voxelotor
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideYou may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
