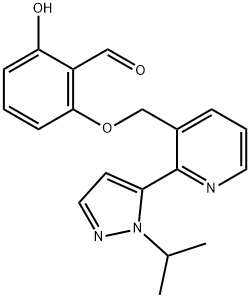
CHEMICAL AND PHYSICAL PROPERTIES
| Boiling Point | 539.2±50.0 |
|---|---|
| Melting Point | 80-82 |
| Solubility | insoluble in water |
| LogP | 2.85 |
| Dissociation Constants | 7.67±0.10 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 337.4 g/mol |
|---|---|
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 337.14264148 g/mol |
| Monoisotopic Mass | 337.14264148 g/mol |
| Topological Polar Surface Area | 77.2 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 434 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Voxelotor is a novel hemoglobin S polymerization inhibitor for the treatment of sickle cell disease. This is a genetically inherited condition most prevalent in the Middle East, Africa, and certain parts of India. Sickle cell disease can lead to excruciating pain, stroke, infection, and various other complications arising from the blockage of blood vessels. Voxelotor was granted accelerated FDA approval on November 25 2019, as it is likely to be a promising treatment for the 100,000 individuals in the U.S. suffering from the disease, in addition to 20 million others worldwide. It was developed by Global Blood Therapeutics, Inc. and is unique from other drugs used to treat sickle cell anemia, such as [hydroxyurea], [L-glutamine], and [crizanlizumab] due to its novel mechanism of action. The EMA approved the use of voxelotor for the treatment of hemolytic anemia associated with sickle cell disease in February 2022.