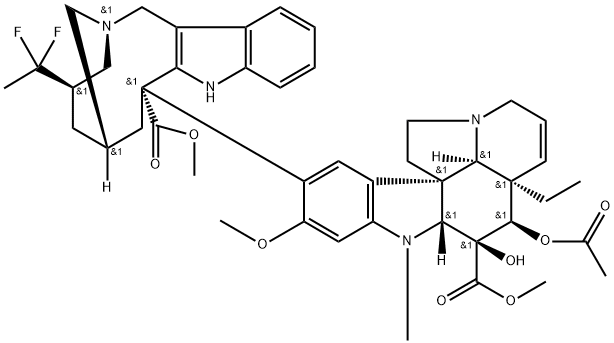
Vinflunine Ditartrate
- CAS NO.:194468-36-5
- Empirical Formula: C49H59F2N4O14
- Molecular Weight: 966.0079664
- MDL number: MFCD12756258
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:18
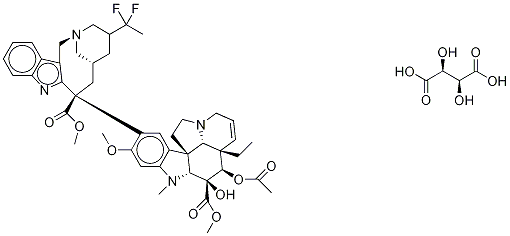
What is Vinflunine Ditartrate?
Chemical properties
White Solid
The Uses of Vinflunine Ditartrate
Vinflunine Ditartrate can be used as Semisynthetic Vinca alkaloid with microtubule destabilizing and antiangiogenic activity; derivative of Vinorelbine.
The Uses of Vinflunine Ditartrate
Vinflunine is a semisynthetic Vinca alkaloid with microtubule destabilizing and antiangiogenic activity. Vinflunine is a derivative of Vinorelbine. Vinflunine is used as an antineoplastic.
Clinical Use
Vinflunine ditartrate is a second generation difluorinated analog of the naturally-occuring substance vinorelbine and it is approved for the treatment of non-small cell lung cancer, metastatic breast cancer and ovarian cancer. Vinflunine, a tubulin polymerization inhibitor, belongs to the vinca alkaloid class of anti-cancer agents. Introduction of the difluoro group of vinflunine dramatically improved antitumor activity of the parent vinorelbine structure. Vinflunine was discovered by Pierre Fabre Laboratories and in 2004 was licensed to Bristol-Myers Squibb for development and commercialization. In 2007, the rights to venflunine were returned to Pierre Fabre which completed its development.
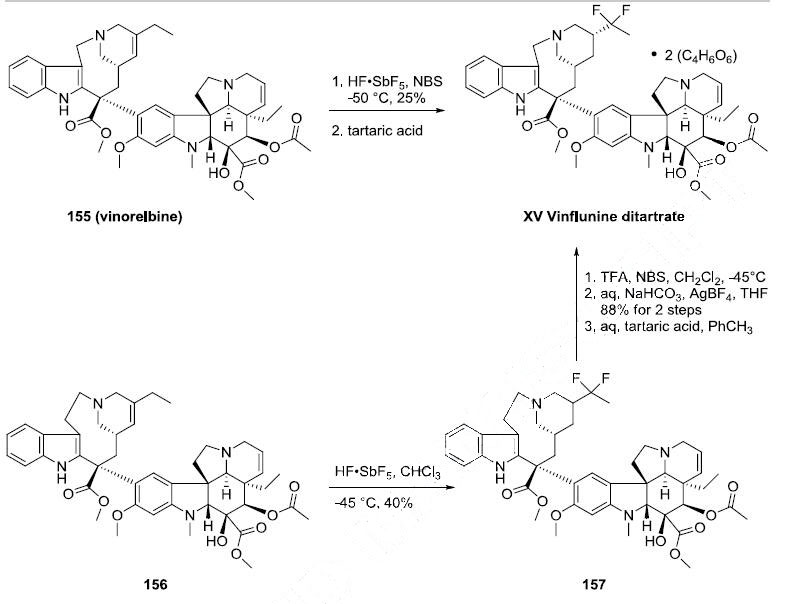
Synthesis
Vinflunine can be prepared directly from vinorelbine (155) through the use of superacid chemistry. Reaction of 155 with antimony pentaflouride in hydrofluoric acid and Nbromosuccinimide followed by treatment with two equivalents of tartaric acid produced vinflunine ditartrate (XV) in 25% yield. An alternative synthesis of vinflunine was realized through reaction of vinblastine or 3?ˉ,4?ˉ-dihydrovinblastine (156) with antimony pentaflouride and hydrofluoric acid in chloroform to give the difluoro alkaloid 157 in 40% yield Ring contraction was effected by reaction with trifluoroacetic acid and N-bromosuccinimide followed by aqueous sodium bicarbonate and silver tetrafluoroborate to give vinflunine in 88% yield. Vinflunine ditartrate (XV) was prepared by treating a solution of vinflunine in toluene with two equivalents of tartaric acid.
Properties of Vinflunine Ditartrate
| Melting point: | 244-246°C (dec) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Light Beige |
Safety information for Vinflunine Ditartrate
Computed Descriptors for Vinflunine Ditartrate
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1