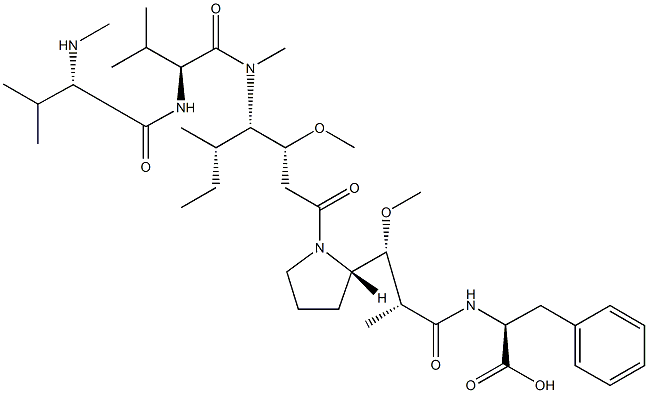
VcMMAE
- CAS NO.:646502-53-6
- Empirical Formula: C68H105N11O15
- Molecular Weight: 1316.63
- MDL number: MFCD25372036
- Update Date: 2026-02-03 17:51:38
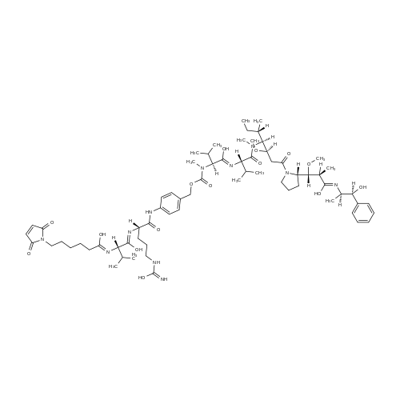
What is VcMMAE?
Description
MC-Val-Cit-PAB-MMAE is a precursor of antibody drug conjugate. It contains a thio reactive maleimidocaproyl (MC) group, a protease-sensitive Val-Cit dipeptide, a PABC linker and a MMAE payload. The MMAE is a synthetic antineoplastic agent. It can be attached to a monoclonal antibody (MAB) which directs it toward cancer cells.
The Uses of VcMMAE
Vedotin is an ultra-high-affinity small organic ligand of fibroblast activation protein used for tumor-targeting applications
What are the applications of Application
VcMMAE is a MMAE derivative with valine-citrulline (Vc) linker. VcMMAE can be used to make antibody drug conjugate. VcMMAE is a anti-mitotic agent, monomethyl auristatin E (MMAE), linked via the lysosomally cleavable dipeptide, valine-citrulline (vc). Monomethyl auristatin E (MMAE) is a synthetic antineoplastic agent. Because of its toxicity, it cannot be used as a drug itself; instead, it is linked to a monoclonal antibody (MAB) which directs it to the cancer cells. In International Nonproprietary Names for MMAE-MAB-conjugates, the name vedotin refers to MMAE plus its linking structure to the antibody. It is a potent antimitotic drug derived from peptides occurring in marine shell-less mollusc Dolabella auricularia called dolastatins which show potent activity in preclinical studies, both in vitro and in vivo, against a range of lymphomas, leukemia and solid tumors. These drugs show potency of up to 200 times that of vinblastine, another antimitotic drug used for Hodgkin lymphoma as well as other types of cancer?
Biological Activity
VcMMAE is an antibody-drug conjugate (ADC) with potent antitumor activity by using the anti-mitotic agent, monomethyl auristatin E (MMAE), linked via the lysosomally cleavable dipeptide, valine-citrulline (vc). As a monoclonal antibody, it acts by binding to the extracellular domain of epidermal growth factor receptor (EGFR) and blocking its interaction with the ligands, thereby inhibiting cellular proliferation.
Properties of VcMMAE
| Boiling point: | 1347.6±65.0 °C(Predicted) |
| Density | 1.196±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in DMSO, DCM, DMF |
| pka | 13.29±0.70(Predicted) |
| form | A solid |
| color | White to off-white |
Safety information for VcMMAE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for VcMMAE
| InChIKey | NLMBVBUNULOTNS-NNNQBTKHNA-N |
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
