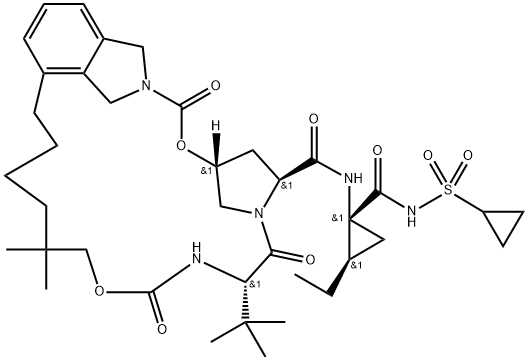
Vaniprevir
- CAS NO.:923590-37-8
- Empirical Formula: C38H55N5O9S
- Molecular Weight: 757.94
- MDL number: MFCD27987919
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
What is Vaniprevir?
Description
Vaniprevir, which was approved in Japan in 2014 and sold under the trade name Vanihep®, is one of several structurallyrelated macrocycles developed for the treatment of patients afflicted with the hepatitis C virus. Specifically, the drug is indicated for patients with untreated, interferon-unresponsive, or relapsed genotype 1 chronic hepatitis C. Similar to asunaprevir (IV), vaniprevir is a NS3/4A protease inhibitor, and thus has a comparable mechanism of action.
The Uses of Vaniprevir
Vaniprevir is an antiviral therapy against hepatitis C.
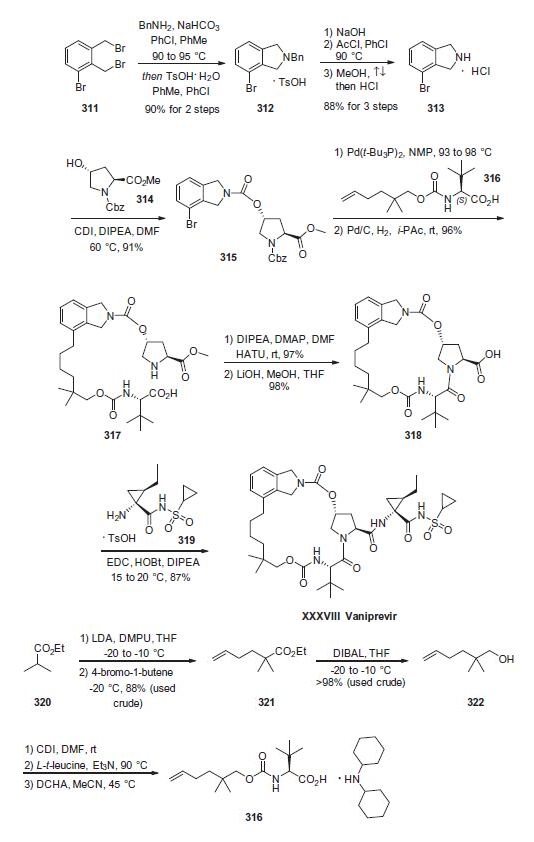
Synthesis
Beginning with commercial bis-benzylbromide 311, subjection to benzylamine under basic conditions followed
by acidification afford the isoindoline as the toluene sulfonic
acid salt 312. This salt was then freebased and acylative removal of
the benzyl protecting group took place through the use of acetyl
chloride. Hydrochloric acid in refluxing methanol removed the
acetamide to liberate indoline 313, which was isolated as the HCl
salt. Next, exposure of 313 to alcohol 314 in the presence of CDI
and warm DMF gave rise to carbamate 315. This was followed by
Heck installation of n-hexenyl fragment 316 and subsequent
hydrogenation of the olefin with concomitant removal of
the benzoyl carbamate protecting group delivered macrocycle precursor
317. Next, an intramolecular lactamization reaction furnished
the macrocyclic system and this was followed by
saponification of the prolinate ester to give 318. This acid was then
coupled with cyclopropylamine 319 under standard
coupling conditions to furnish vaniprevir in 87% yield.
The preparation of hexenyl fragment 316 started
with the lithiation of commercially available ethyl isobutyrate
(320) and alkylative quench with 1-bromo-4-butene to provide
hexenyl ester 321. Next, DIBAL reduction followed by CDI-mediated
carbamate formation with L-t-leucine and subsequent treatment
with dicyclohexylamine (DCHA) furnished the key hexenyl
fragment 316.
The assembly of cyclopropylamine 319 stems from
cyclopropyl fragment 34, whose synthesis was described in
Scheme 5. Hydrogenation of this system to saturate the double
bond was followed by treatment with toluenesulfonic acid to
remove the Boc group, furnishing the tosylate salt in good yield
for the two step sequence.

Properties of Vaniprevir
| Melting point: | 175-177 °C |
| Density | 1.33 |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 4.58±0.40(Predicted) |
| color | White to off-white |
Safety information for Vaniprevir
Computed Descriptors for Vaniprevir
New Products
(E)-1-Ethoxyethene-2-boronic Acid Pinacol Ester 1,3-Diethyl-1,3-Diphenylurea 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,4-dihydroxybenzaldehyde 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 1,3-Di Iodo Benzene Methyl 2-oxo-2,3-dihydrobenzo[d]oxazole-7-carboxylate 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 3-Hydroxy-4-nitrobromobenzene 2-Ethyl-1,4-diaminobenzene 2-Ethylhexyl 4-aminobenzoate Thio AcetamideRelated products of tetrahydrofuran
You may like
-
 MFCD22560726 98%View Details
MFCD22560726 98%View Details
MFCD22560726 -
 3-Hydroxy-4-nitrobromobenzene 98%View Details
3-Hydroxy-4-nitrobromobenzene 98%View Details
2768-84-0 -
 4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 98%View Details
4-(2-Aminoethyl)-7-hydroxy-2H-chromoen-2-one 98%View Details
1234064-08-4. -
 2-Ethyl-1,4-diaminobenzene 98%View Details
2-Ethyl-1,4-diaminobenzene 98%View Details
MFCD19203885 -
 85-81-4 6-Methoxy-8-nitroquinoline 98%View Details
85-81-4 6-Methoxy-8-nitroquinoline 98%View Details
85-81-4 -
 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 98%View Details
2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 98%View Details -
 Hot Sales:Thio AcetamideView Details
Hot Sales:Thio AcetamideView Details
62-55-5 -
 CosmoticsView Details
CosmoticsView Details
26218-04-2