Urea
Synonym(s):Urea;Carbamide;NBK;Urea solution;Ureum
- CAS NO.:57-13-6
- Empirical Formula: CH4N2O
- Molecular Weight: 60.06
- MDL number: MFCD00008022
- EINECS: 200-315-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
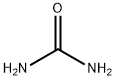
What is Urea?
Description
Urea is a stable highly water-soluble compound
of high nitrogen content (47%), with good storage
properties that make it the most commonly used nitrogen
fertilizer. The synthesis process has remained essentially
unchanged since it was first developed by the BASF
Corporation in 1922. In this process, liquid ammonia
is reacted with carbon dioxide to produce ammonium
carbamate, which is then dehydrated to form urea. The
reactions are:
2NH3 + CO2 === NH2·CO2·NH4
NH2·CO2·NH4 === (NH2)2CO + H2O
Chemical properties
Urea,CO(HN2)2, also known as carbamide, is a white crystalline powder that has a melting point of l32.7 °C (270 °F). It is a natural product of animal protein metabolism and is the chief nitrogen constituent of urine. Commercially, urea is produced by the reaction of ammonia and carbon dioxide. It is soluble in water, alcohol, and benzene.
Occurrence
The compound was discovered by Hilaire Rouelle in 1773 as a constituent of urine.
History
Urea has the distinction of being the first synthesized organic compound. Until the mid-18th century, scientists believed organic compounds came only from live plants and animals. The first serious blow to the theory of vitalism, which marked the beginning of modern organic chemistry, occurred when Friedrich W?hler (1800 1882) synthesized urea from the two inorganic substances, lead cyanate and ammonium hydroxide: Pb(OCN)2 + 2NH4OH→2(NH2)2CO + Pb(OH)2. W?hler's discoveries on urea occurred while he was studying cyanates; he was attempting to synthesize ammonium cyanate when he discovered crystals of urea in his samples. He first prepared urea in 1824, but he did not identify this product and report his findings until 1828. W?hler's synthesis of urea signaled the birth of organic chemistry.
Urea Applications
Urea is a vital physiological regulator of nitrogen excretion in mammals, synthesized in the liver from protein catabolism and excreted in urine. It also occurs naturally in the skin and serves multiple functions in various applications.
In the cosmetic industry, urea is incorporated for its moisturizing, desquamating, anti-microbial, and buffering properties. Unlike a humectant, urea is considered a 'true' moisturizer as it attracts and retains moisture in the stratum corneum, facilitating the natural exfoliation of keratinocytes by dissolving intercellular cement. Its anti-microbial properties help inhibit the growth of microorganisms, potentially contributing to a product's preservative system. Urea's buffering action is due to its ability to regulate the hydrolipid mantle, and it also enhances the penetration and absorption of other active ingredients, relieving itchiness and leaving the skin soft and supple. With anti-inflammatory, antiseptic, and deodorizing actions, urea helps protect the skin's surface and maintain healthy skin conditions. Studies indicate that urea does not induce photoallergy, phototoxicity, or sensitization, and the safest concentration for use in skin care is between 2 and 8 percent. However, high concentrations can be unstable and potentially irritating, and acidic urea solutions may cause burning or stinging sensations.
The Uses of Urea
The primary use of urea is as a nitrogen source in fertilizers, with about 90% of the urea production being used for this purpose. Urea's high nitrogen content (46%) makes it a concentrated source for adding fixed nitrogen to soils. Another use of urea is for resins, which are used in numerous applications including plastics, adhesives, moldings, laminates, plywood, particleboard, textiles, and coatings.
Indications
Urea is used topically for debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses, as well as damaged, devitalized and ingrown nails.
Background
In the liver, a compound is formed from ammonia, which is produced by the deamination of amino acids. This compound is the principal end product of protein catabolism and makes up approximately half of the total urinary solids.
Definition
A white crystalline compound made from ammonia and carbon dioxide. It is used in the manufacture of urea–formaldehyde (methanal) resins. Urea is the end product of metabolism in many animals and is present in urine.
Production Methods
Urea is an important industrial compound. The synthesis of urea was discovered in 1870.Commercial production of urea involves the reaction of carbon dioxide and ammonia at highpressure and temperature to produce ammonium carbamate. Ammonium carbamate is thendehydrated to produce urea (Figure 96.1). The reaction uses a molar ratio of ammonia tocarbon dioxide that is approximately 3:1 and is carried out at pressures of approximately 150atmospheres and temperatures of approximately 180°C.
Preparation
All current processes for the manufacture of urea are based on the reaction of ammonia and carbon dioxide to form ammonium carbamate which is simultaneously dehydrated to urea:
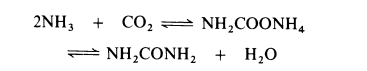
The dehydration of ammonium carbamate is appreciable only at temperatures above the melting point (about 150??C) and this reaction can only proceed if the combined partial pressure of ammonia and carbon dioxide exceeds the dissociation pressure of the ammonium carbamate (about 10 MPa at 160??C and about 30 MPa at 200??C). Thus commercial processes are operated in the liquid phase at 160-220??C and 18-35 MPa (180-350 atmospheres). Generally, a stoichiometric excess of ammonia is employed, molar ratios of up to 6: 1 being used. The dehydration of ammonium carbamate to urea proceeds to about 50-65% in most processes.
brand name
Ureaphil (Hospira).
Biological Functions
The use of urea (Ureaphil, Urevert) has declined in recent years owing both to its disagreeable taste and to the increasing use of mannitol for the same purposes. When used to reduce cerebrospinal fluid pressure, urea is generally given by intravenous drip. Because of its potential to expand the extracellular fluid volume, urea is contraindicated in patients with severe impairment of renal, hepatic, or cardiac function or active intracranial bleeding.
General Description
Solid odorless white crystals or pellets. Density 1.335 g /cc. Noncombustible.
Air & Water Reactions
Water soluble.
Reactivity Profile
Urea is a weak base. Reacts with hypochlorites to form nitrogen trichloride which explodes spontaneously in air [J. Am. Chem. Soc. 63:3530-32]. Same is true for phosphorus pentachloride. Urea reacts with azo and diazo compounds to generate toxic gases. Reacts with strong reducing agents to form flammable gases (hydrogen). The heating of improper stoichiometric amounts of Urea and sodium nitrite lead to an explosion. Heated mixtures of oxalic acid and Urea yielded rapid evolution of gases, carbon dioxide, carbon monoxide and ammonia (if hot, can be explosive). Titanium tetrachloride and Urea slowly formed a complex during 6 weeks at 80°C., decomposed violently at 90°C., [Chem. Abs., 1966, 64, 9219b]. Urea ignites spontaneously on stirring with nitrosyl perchlorate, (due to the formation of the diazonium perchlorate). Oxalic acid and Urea react at high temperatures to form toxic and flammable ammonia and carbon monoxide gasses, and inert CO2 gas [Von Bentzinger, R. et al., Praxis Naturwiss. Chem., 1987, 36(8), 41-42].
Health Hazard
May irritate eyes.
Fire Hazard
Behavior in Fire: Melts and decomposes, generating ammonia.
Agricultural Uses
Fertilizer, Fungicide: Used in fertilizers and animal feeds, as a fungicide, in the manufacture of resins and plastics, as a stabilizer in explosives and in medicines, and others. Urea is used to protect against frost and is used in some pesticides as an inert ingredient as a stabilizer, as an inhibitor and as an intensifier for herbicides. Registered for use in EU countries . Registered for use in the U.S.
Trade name
PRESPERSION, 75 UREA®; SUPERCEL 3000®; UREAPHIL®; UREOPHIL®; UREVERT®; VARIOFORM II®
Biochem/physiol Actions
Urea solution is primarily used for protein denaturation. It also increases solubility of hydrocarbons and reduce micelle formation. Urea solution at high concentration leads to the destabilization of amyloid β16?22 oligomers.
Pharmacokinetics
Urea is a keratolytic emollient that works to treat or prevent dry, rough, scaly, itchy skin.
Safety Profile
Moderately toxic by intravenous and subcutaneous routes. Human reproductive effects by intraplacental route: ferthty effects. Experimental reproductive effects. Human mutation data reported. A human skin irritant. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Reacts with sodium hypochlorite or calcium hypochlorite to form the explosive nitrogen trichloride. Incompatible with NaNO2, P2Cl5, nitrosyl perchlorate. Preparation of the 15N-labeled urea is hazardous. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Urea is used in ceramics, cosmetics, paper processing; resins, adhesives, in animal feeds; in the manufacture of isocyanurates; resins, and plastics; as a stabilizer in explosives; in medicines; anticholelithogenic, and others.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Terrestrial Fate
Urea is expected to have very high mobility in soil. Urea is not expected to volatilize from dry soil surfaces based on its vapor pressure. Various field and laboratory studies have demonstrated that urea degrades rapidly in most soils. Urea is rapidly hydrolyzed to ammonium ions through soil urease activity, which produces volatile gases, that is, ammonia and carbon dioxide. However, the rate of hydrolysis can be much slower, depending on the soil type, moisture content, and urea formulation.
Aquatic Fate
Urea is not expected to adsorb to suspended solids and sediments. Volatilization from water surfaces is not expected. Urea is rapidly hydrolyzed to ammonia and carbon dioxide in environmental systems by the extracellular enzyme urease, which originates from microorganisms and plant roots.
Atmospheric Fate
According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere, urea, which has a vapor pressure of 1.2×10-5mm Hg at 251°C, will exist in both the vapor and particulate phases in the ambient atmosphere. Vapor-phase urea is degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 9.6 days.
Metabolism
The high analysis and good handling properties of urea have made it the leading nitrogen fertilizer, both as a source of nitrogen alone or when compounded with other materials in mixed fertilizers. Although an excellent source of nitrogen, urea can present problems unless properly managed; due to its rapid hydrolysis to ammonia, significant volatilization loss of this may occur if prilled or granular urea is applied to and left on the soil surface without timely incorporation. Mixtures of urea and ammonium nitrate for use in mixed fertilizers are also more highly hygroscopic than ammonium nitrate itself.
Storage
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from oxidizers. Where possible, automatically transfer material fromstorage containers to process containers.
Purification Methods
Crystallise urea twice from conductivity water using centrifugal drainage and keeping the temperature below 60o. The crystals are dried under vacuum at 55o for 6hours. Levy and Margouls [J Am Chem Soc 84 1345 1962] prepared a 9M solution in conductivity water (keeping the temperature below 25o) and, after filtering through a medium-porosity glass sinter, added an equal volume of absolute EtOH. The mixture was set aside at -27o for 2-3 days and filtered cold. The precipitate was washed with a small amount of EtOH and dried in air. Crystallisation from 70% EtOH between 40o and -9o has also been used. Ionic impurities such as ammonium isocyanate have been removed by treating the concentrated aqueous solution at 50o with Amberlite MB-1 cation-and anion-exchange resin, and allowing it to crystallise on evaporation. [Benesch et al. J Biol Chem 216 663 1955.] It can also be crystallised from MeOH or EtOH, and is dried under vacuum at room temperature. [Beilstein 3 H 42, 3 I 19, 3 II 35, 3 III 80.]
Toxicity evaluation
The primary mechanism of toxicity for Urea appears to be inhibition of the citric acid cycle. It leads to blockade of electron transport and a decrease in energy production and cellular respiration, which leads to convulsions.
Incompatibilities
Violent reaction with strong oxidizers, chlorine, permanganates, dichromates, nitrites, inorganic chlorides; chlorites, and perchlorates. Contact with hypochlorites can result in the formation of explosive compounds.
Waste Disposal
Controlled incineration in equipment containing a scrubber or thermal unit to reduce nitrogen oxide emissions.
Properties of Urea
| Melting point: | 132-135 °C(lit.) |
| Boiling point: | 332.48°C (estimate) |
| Density | 1.335 g/mL at 25 °C(lit.) |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | n |
| storage temp. | 2-8°C |
| solubility | H2O: 8 M at 20 °C |
| form | powder |
| appearance | white crystals or powder |
| pka | 0.10(at 25℃) |
| color | white |
| Specific Gravity | 1.335 |
| Odor | almost odorless |
| PH | 8.0-10.0 (20℃, 8M in H2O) |
| Water Solubility | 1080 g/L (20 ºC) |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,9867 |
| BRN | 635724 |
| Dielectric constant | 3.5(Ambient) |
| Stability: | Substances to be avoided include strong oxidizing agents. Protect from moisture. |
| CAS DataBase Reference | 57-13-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Urea(57-13-6) |
| EPA Substance Registry System | Urea (57-13-6) |
Safety information for Urea
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H320:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Urea
| InChIKey | XSQUKJJJFZCRTK-UHFFFAOYSA-N |
Urea manufacturer
New Products
4-Iodo-3,5-dimethylbenzonitrile 2-fluoro-4-iodoaniline 2-(3-Methyl-1,2,4-oxadiazol-5-yl)acetic acid (as potassium salt) 6,6'-methylenebis(9-methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-2,3-dihydro-1H-carbazol-4(9H)-one) 3-Bromo-2-fluorobenzoic acid Quinuclidine-4-carbonitrile N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 1,4-bis(methylsulfonyl)butane 4-Cyano-N-Methacryloyl-3-Trifluoromethyl Aniline 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzeneRelated products of tetrahydrofuran
![Urea, N,N'-bis[[(5S)-2-oxo-3-[4-(3-oxo-4-Morpholinyl)phenyl]-5-oxazolidinyl]Methyl]-](https://img.chemicalbook.in/CAS/20150408/GIF/1365267-35-1.gif)



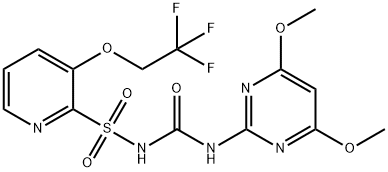

You may like
-
 Urea 98%View Details
Urea 98%View Details -
 Urea 99%View Details
Urea 99%View Details -
 Urea 99%View Details
Urea 99%View Details -
 Urea 98%View Details
Urea 98%View Details -
 Urea, AR 99%View Details
Urea, AR 99%View Details -
 Urea (SQ) CAS 57-13-6View Details
Urea (SQ) CAS 57-13-6View Details
57-13-6 -
 Urea (ER ACS) CAS 57-13-6View Details
Urea (ER ACS) CAS 57-13-6View Details
57-13-6 -
 Technical Grade Urea, >99%, Packaging Size: 25 kgView Details
Technical Grade Urea, >99%, Packaging Size: 25 kgView Details
57-13-6
