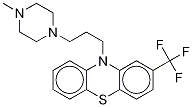
Triflupromazine hydrochloride
Synonym(s):N,N-Dimethyl-2-(trifluoromethyl)-10H-phenothiazine-10-propanamine monohydrochloride
- CAS NO.:1098-60-8
- Empirical Formula: C18H20ClF3N2S
- Molecular Weight: 388.88
- MDL number: MFCD00058103
- EINECS: 214-149-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-26 13:56:28
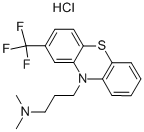
What is Triflupromazine hydrochloride?
Description
Triflupromazine is a phenothiazine with diverse biological activities. It binds to muscarinic receptors in isolated rat corpus striatum (IC50 = 100 μM in a radioligand binding assay). Triflupromazine inhibits serotonin (5-HT) uptake by isolated rat brainstem synaptosomes (IC50 = 0.8 μM). It inhibits T. cruzi infection in mouse peritoneal macrophages when used at a concentration of 12.5 μM. Triflupromazine is active against S. aureus, shigellae, and vibrios (MICs = 2-100 μg/ml) in vitro and is protective against S. typhimurium infection in mice when administered at a dose of 30 μg per animal. Formulations containing triflupromazine were previously used as antipsychotics.
Originator
Vesprin,Squibb,US,1957
The Uses of Triflupromazine hydrochloride
analgesic, antiinflammatory, antipyretic, COX-II inhibitor
What are the applications of Application
Triflupromazine hydrochloride is a neurology research compound
Manufacturing Process
Approximately 3.8 grams of sodamide is freshly prepared from 2.25 grams of
sodium, 90 grams of liquid ammonia and a catalytic trace of ferric nitrate. The
ammonia is allowed to evaporate. A solution of 19.1 grams of 2-trifluoromethylphenothiazine (prepared by the Bernthsen thionation of 3-
trifluoromethyldiphenylamine) in 160 ml of dry benzene is added to the
reaction flask followed by 18 grams of 3-chloro-1-dimethylaminopropane. The
reaction mixture is heated at reflux for 20 hours. After washing the cooled
mixture with 130 ml of water, the organic layer is extracted with several
portions of dilute hydrochloric acid. The acid extracts are combined and
neutralized with ammonium hydroxide solution. The oily free base is extracted
into benzene and purified by distillation to give 19.6 grams of 10-(3'-
dimethylaminopropyl)-2-trifluoromethylphenothiazine, boiling point 177° to
181°C at 1 mm. The free base (7 grams) is converted to the hydrochloride
salt by reacting an alcoholic solution of the base with hydrogen chloride gas.
Evaporation of the volatiles in vacuo leaves an amorphous solid which is
recrystallized from ethanol/ether to pink crystals, MP 173° to 174°C, the
hydrochloride salt of the free base prepared above.
brand name
Vesprin (Bristol-Myers Squibb).
Therapeutic Function
Tranquilizer
General Description
Triflupromazinehydrochloride, 10-[3-(dimethylamino)propyl]-2-(trifluoromethyl)phenothiazine monohydrochloride (Vesprin), has agreater milligram potency as an antipsychotic, higher EPS,but lower sedative and hypotensive effects than chlorpromazine.The 2-CF3 versus the 2-Cl is associated with thesechanges. Overall, the drug has uses analogous to those ofchlorpromazine.
Properties of Triflupromazine hydrochloride
| Melting point: | 174.0 to 179.0 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| Water Solubility | Soluble in water |
| solubility | DMF: 5mg/mL; DMSO: 3mg/mL; Ethanol: 5mg/mL; Ethanol:PBS (pH 7.2) (1:10): 0.09mg/mL |
| form | neat |
| color | White to Light yellow |
| λmax | 305nm(H2O)(lit.) |
| Merck | 14,9685 |
| BRN | 3801519 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 1098-60-8 |
Safety information for Triflupromazine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Triflupromazine hydrochloride
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 1098-60-8 TRIFLUPROMAZINE HCL IP/USP 98%View Details
1098-60-8 TRIFLUPROMAZINE HCL IP/USP 98%View Details
1098-60-8 -
 Triflupromazine Hydrochloride CAS 1098-60-8View Details
Triflupromazine Hydrochloride CAS 1098-60-8View Details
1098-60-8 -
 Triflupromazine hydrochloride 95% CAS 1098-60-8View Details
Triflupromazine hydrochloride 95% CAS 1098-60-8View Details
1098-60-8 -
 Triflupromazine hydrochloride CAS 1098-60-8View Details
Triflupromazine hydrochloride CAS 1098-60-8View Details
1098-60-8 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
