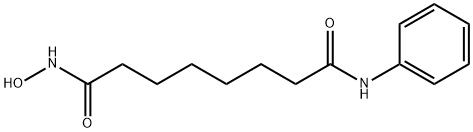
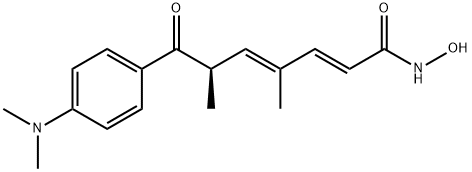
TRICHOSTATIN A
Synonym(s):[R-(E,E)]-7-[4-(Dimethylamino)phenyl]-N-hydroxy-4,6-dimethyl-7-oxo-2,4-heptadienamide;4,6-Dimethyl-7-[ p-dimethylaminophenyl]-7-oxahepta-2,4-dienohydroxamic Acid, TSA, HDAC Inhibitor IX;Trichostatin A;Trichostatin A, Streptomyces sp. - CAS 58880-19-6 - Calbiochem;TSA
- CAS NO.:58880-19-6
- Empirical Formula: C17H22N2O3
- Molecular Weight: 302.37
- MDL number: MFCD03848392
- EINECS: 611-758-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-29 18:33:56
What is TRICHOSTATIN A?
Description
Trichostatin A (58880-19-6) is a potent and selective histone deacetylase (HDAC) inhibitor (Ki = 3.4 nM). Induces reversion of ras transformed cells to normal morphology. Trichostatin A induces dedifferentiation of primordial germ cells into embryonic germ cells. Cell permeable and active in vivo.
Chemical properties
Solid
The Uses of TRICHOSTATIN A
Trichostatin A is a known inhibitor of fibrosis in vitro and in vivo, and is used as an anticancer agent. Potent differentiation inducer of friend leukemic cells.
The Uses of TRICHOSTATIN A
Trichostatin A is a histone deacetylase inhibitor that enhances the cytotoxic efficacy of anticancer drugs that target DNA. Trichostatin A displays antifungal, antiprotozoan and antitumour activity
The Uses of TRICHOSTATIN A
Trichostatin A, ready-made solution has been used:
- as a histone deacetylase inhibitor to study its effect on transcriptome changes by stem cell testing
- to inhibit histone deacetylase (HDAC) class I, II, or III in primary pituitary cell cultures and to investigate insulin control of endogenous human growth hormone gene (hGH)
- to treat cells for the HDAC inhibition experiments
What are the applications of Application
Trichostatin A is a potent and non-competitive reversible inhibitor of HDAC in LNCaP human prostate cancer cells, as well as in MON and HeLa cells.
Definition
ChEBI: Trichostatin A is an antibiotic antifungal agent, a trichostatin and a hydroxamic acid. It has a role as a bacterial metabolite, a geroprotector and an EC 3.5.1.98 (histone deacetylase) inhibitor. It is functionally related to a (R)-trichostatic acid.
General Description
Trichostatin A is a compound of primary hydroxamic acid.
Biological Activity
Selective and potent inhibitor of histone deacetylase (K i = 3.4 nM). Active in vivo . Potential anti-cancer agent. Induces accelerated dedifferentiation of primordial germ cells (PGCs) into embryonic germ (EG) cells.
Biochem/physiol Actions
Inhibits histone deacetylase at nanomolar concentrations; resultant histone hyperacetylation leads to chromatin relaxation and modulation of gene expression. May be involved in cell cycle progression of several cell types, inducing cell growth arrest at both G and G/M phases; may induce apoptosis. Enhances the efficacy of anticancer agents that target DNA.
Storage
-20°C (desiccate)
References
1) Yoshida et al. (1990), Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A ; J. Biol. Chem., 265 17174 2) Futamura et al. (1995), Trichostatin A inhibits both ras-induced neurite outgrowth of PC12 cells and morphological transformation of NIH3T3 cells; Oncogene, 10 1119 3) Durcova-Hills et al. (2008), Reprogramming Primordial Germ Cells into Pluripotent Stem Cells; PLoS-One, 3 e3531
Properties of TRICHOSTATIN A
| Melting point: | 140-143℃ |
| alpha | D20.5 +62.8° ±1.1° (c = 1.007 in ethanol) |
| Density | 1.139 |
| refractive index | 136 ° (C=0.3, MeOH) |
| storage temp. | -20°C |
| solubility | ethanol: 1 mg/mL |
| form | Off-white lyophilized solid |
| pka | 8.93±0.23(Predicted) |
| color | Tan |
| Merck | 14,9649 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for TRICHOSTATIN A
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TRICHOSTATIN A
| InChIKey | ZWGICMNKJBUOHG-RCXLNATQSA-N |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 58880-19-6 Trichostatin A 98%View Details
58880-19-6 Trichostatin A 98%View Details
58880-19-6 -
 Trichostatin A CAS 58880-19-6View Details
Trichostatin A CAS 58880-19-6View Details
58880-19-6 -
 Trichostatin A CAS 58880-19-6View Details
Trichostatin A CAS 58880-19-6View Details
58880-19-6 -
 Trichostatin A, Streptomyces sp. CASView Details
Trichostatin A, Streptomyces sp. CASView Details -
 Trichostatin A, Streptomyces sp. CAS 58880-19-6View Details
Trichostatin A, Streptomyces sp. CAS 58880-19-6View Details
58880-19-6 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8