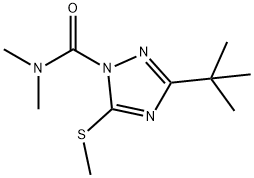
TRIAZAMATE
Synonym(s):Ethyl (3-tert-butyl-1-dimethylcarbamoyl-1H-1,2,4-triazol-5-ylthio)acetate;Ethyl 2-{{1-[(dimethylamino)carbonyl]-3-(1,1-dimethylethyl)-1H-1,2,4-triazol-5-yl}thio}acetate
- CAS NO.:112143-82-5
- Empirical Formula: C13H22N4O3S
- Molecular Weight: 314.4
- MDL number: MFCD01632354
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:27:03
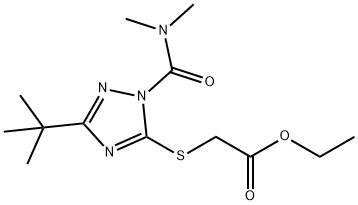
What is TRIAZAMATE?
The Uses of TRIAZAMATE
Triazamate is used for the control of aphids by foliar application on a wide variety of crops. It is suitable for inclusion in integrated pest management because it is minimally toxic to beneficial insects.
The Uses of TRIAZAMATE
Pesticide.
The Uses of TRIAZAMATE
Triazamate is used in controlling unpleasant pests.Used in preparation of Thiazole Manganese Zinc compounds and compounds thereof.
Definition
ChEBI: A triazole insecticide that is 1H-1,2,4-triazole which is substituted at positions 1, 3, and 5 by N,N-dimethylaminocarbonyl, tert-butyl, and (2-ethoxy-2-oxoethyl)sulfanediyl groups, resp ctively.
General Description
Triazamate is a carbamoyl triazole compound, widely used as an insecticide and its mode of action involves the inhibition of cholinesterase in insect pests.
Metabolic pathway
There is only a limited amount of information on the fate of triazamate currently in the public domain. Metabolism in sugar beet and apple has been reported. Animal metabolism studies have been conducted but details are not available. Triazamate is very rapidly metabolised by hydrolysis, decarbamoylation and further metabolism in all biological systems studied (PM).
Degradation
Triazamate is stable under normal conditions and in solution at pH 7
and below. Its DT50 values in buffers are, at pH 5, 7 and 9, 220 days,
49 hours and 1 hour, respectively (PM). Base-catalysed hydrolysis should
initially afford triazamate acid (2) by carboxyl ester cleavage (see
Scheme 1).
The DTM of triazamate on aqueous photolysis at pH 7 is 301 days
(PM)
Properties of TRIAZAMATE
| Melting point: | 60° |
| Boiling point: | 280°C (rough estimate) |
| Density | 1.2357 (rough estimate) |
| vapor pressure | 1.6 x l0-4 Pa (25 °C) |
| refractive index | 1.6200 (estimate) |
| Flash point: | 189℃ |
| pka | 0.48±0.50(Predicted) |
| form | neat |
| Water Solubility | 433 mg l-1(25 °C) |
| BRN | 8422595 |
| EPA Substance Registry System | Triazamate (112143-82-5) |
Safety information for TRIAZAMATE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TRIAZAMATE
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![5-ethyl-6-octyl-[1,2,3]triazolo[1,5-a]pyriMidin-7-aMine](https://img.chemicalbook.in/CAS/20150408/GIF/865318-97-4.gif)
![[4-chloro-2-(hydroxymethyl)phenoxy]acetic acid](https://img.chemicalbook.in/CAS/GIF/6386-63-6.gif)
You may like
-
 Triazamate CAS 112143-82-5View Details
Triazamate CAS 112143-82-5View Details
112143-82-5 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
