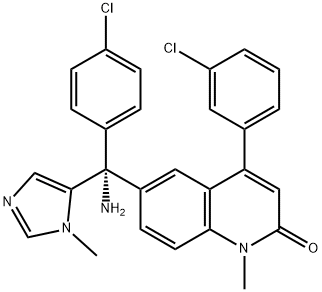
Tipifarnib
Synonym(s):(R)-(+)-R 115777;6-[(R)-Amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone;R115777
- CAS NO.:192185-72-1
- Empirical Formula: C27H22Cl2N4O
- Molecular Weight: 489.4
- MDL number: MFCD07772347
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
What is Tipifarnib?
Chemical properties
Off-White to Pale Beige Solid
The Uses of Tipifarnib
A farnesyltransferase inhibitor. Sensitizes human multiple myeloma cell to proteasome inhibition by blocking degradation of bortezomib(B675700)-induced aggresomes. Also shown to inhibit the growth of myeloid leukemia cell lines and primary leukemia cells by inducing apoptosis and cell-cycle blockage when combined with rapamycin(R124000).
The Uses of Tipifarnib
A farnesyltransferase inhibitor. Sensitizes human multiple myeloma cell to proteasome inhibition by blocking degradation of bortezomib(B675700)-induced aggresomes. Also shown to inhibit the growth of myeloid leukemia cell lines and primary leukemia cells by inducing apoptosis and cell-cycle blockage when combined with rapamycin(R124000).
What are the applications of Application
Tipifarnib is an inhibitor of the kappa B-Ras peptide
Definition
ChEBI: Tipifarnib is a quinolone that is 1-methylquinolin-2-one which carries a 3-chlorophenyl and an amino(4-chlorophenyl)(1-methyl-imidazol-5-yl)methyl groups at the 4 and 6 positions, respectively (the R-isomer). It has a role as an antineoplastic agent, an EC 2.5.1.58 (protein farnesyltransferase) inhibitor and an apoptosis inducer. It is a quinolone, a member of monochlorobenzenes, a member of imidazoles and a primary amino compound.
General Description
Pre-clinical studies show that tipifarnib has antiproliferative effects on pancreatic cancer cell lines at clinically relevant concentrations (concentration that inhibits 50% growth [IC50] from 9.5 to 500?nmol/L). It also exhibits marked growth retardation and antiangiogenic effects in a pancreatic cancer xenograft model.
Biological Activity
tipifarnib (also known as zarnestra or r115777), an orally bioavailable quinolone analog of imidazole heterocyclics, is a potent and specific nonpeptidomimetic competitive inhibitor of farnesyltransferase (ftase), an enezyme mediating post-translational farnesylation of multiple protein substrates involved in tumor cell proliferation. it has demonstrated inhibition of growth and proliferation of a broad range of human tumor models (either wild-type or mutated ras) via cytostatic rather than cytotoxic activity both in vitro and in vivo. it cell-type dependently induces apoptosis in some neoplastic cell lineages other than acute myeloid leukemia (aml), including multiple myeloma (mm) cell lines and mm cultures from patients.p.k. epling-burnett and thomas p. loughran jr. suppression of farnesyltransferase activity in acute myeloid leukemia and myelodysplastic syndrome: current understanding and recommended use of tipifarnib. expert opin investig drugs. 2010; 19(5): 689-698jean-pierre armand, alan k. burnett, johannes drach, jean-luc harousseau, bob lowenberg and jesus san miguel. the emerging role of targeted therapy for hematologic malignancies: update on bortezomib and tipifarnib. the oncologist 2007, 12:281-290elzbieta izbicka, david campos, gilbert carrizales and amita patnaik. biomarkers of anticancer activity of r115777 (tipifarnib, zarnestra) in human breast cancer models in vitro. anticancer research 2005; 25: 3215-3224
Biochem/physiol Actions
Tipifarnib (R115777) is an orally active and potent farnesyltransferase (FTase) inhibitor that exhibits potent anti-tumorigenic effects. Tipifarnib mechanism of action is not fully understand.
Properties of Tipifarnib
| Melting point: | 211-213°C (dec.) |
| Boiling point: | 681.7±55.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 7.20±0.10(Predicted) |
| color | white to beige |
| optical activity | [α]/D 17 to 24°, c = 1 in methanol |
Safety information for Tipifarnib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tipifarnib
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![FARNESENE (MIXTURE OF ISOMERS)[FOR PERFUMERY]](https://img.chemicalbook.in/)




You may like
-
 Tipifarnib CAS 192185-72-1View Details
Tipifarnib CAS 192185-72-1View Details
192185-72-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
