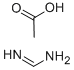
Thiourea dioxide
Synonym(s):Aminoiminomethanesulfinic acid;FormamidinesulfInic acid, Thiourea dioxide;Thiourea dioxide
- CAS NO.:1758-73-2
- Empirical Formula: CH4N2O2S
- Molecular Weight: 108.12
- MDL number: MFCD00002397
- EINECS: 217-157-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:19
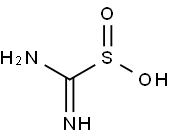
What is Thiourea dioxide?
Chemical properties
white crystalline powder
The Uses of Thiourea dioxide
Convenient reagent for the reduction of ketones to secondary alcohols.
Thiourea dioxide is an effective bleach when used alone or when used after hydrogen peroxide in a full bleaching process (Duffield, 1986; Cegarra et al, 1988). Bleaching with thiourea dioxide is not common practice but it is effective when used alone, and the process compares favorably with hydrogen peroxide bleaching. A formulation can include a commercial thiourea dioxide product, wetting agent and EDTA sequestering agent. Reductive bleaching is carried out at pH 7.0 at 70°C for 60 min (Duffield, 1986).

General Description
A white or light-yellow odorless crystalline powder. Mp:126°C. Soluble in water (27 g / L at room temperature). Decomposes exothermically at temperatures above 126°C with the emission of noxious gases (sulfur oxides, ammonia, carbon monoxide, nitrogen oxides and hydrogen sulfide) and carbon dioxide. Extended exposure to temperatures above 50°C and moisture may cause visible decomposition. Irritating to skin and mucous membranes. Corrosive to eye tissue. Used in leather processing, the paper industry, photographic industry, and in textile processing as a bleaching agent.
Air & Water Reactions
Soluble in water
Reactivity Profile
Thiourea dioxide is a reducing agent and a derivative of sulfinic acid (a weak inorganic acid). Decolorizes and bleaches materials by chemical reduction. Stable under normal temperatures and pressures. May decompose on exposure to moist air or water. Incompatible with strong oxidizing agents, strong bases. Aqueous solutions are acidic and corrosive.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Purification Methods
Dissolve it in five parts of aqueous 1:1% NaHSO3 at 60-63o (charcoal), then allow it to crystallise slowly, with agitation, at 10o. Filter and dry it immediately at 60o [Koniecki & Linch Anal Chem 30 1134 1958]. [Beilstein 3 I 36, 3 IV 145.]
Properties of Thiourea dioxide
| Melting point: | 124-127 °C (dec.)(lit.) |
| Boiling point: | 355.3±25.0 °C(Predicted) |
| Density | 1.68 |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.6550 (estimate) |
| storage temp. | 2-8°C |
| solubility | 27g/l |
| form | Crystalline Powder |
| pka | 2.40±0.10(Predicted) |
| color | White |
| PH | 4 (10g/l, H2O, 20℃) |
| Water Solubility | 30 g/L (20 ºC) |
| Sensitive | Moisture Sensitive |
| BRN | 506653 |
| CAS DataBase Reference | 1758-73-2(CAS DataBase Reference) |
| EPA Substance Registry System | Methanesulfinic acid, aminoimino- (1758-73-2) |
Safety information for Thiourea dioxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H251:Self-heating substances and mixtures H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P235:Keep cool. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Thiourea dioxide
| InChIKey | FYOWZTWVYZOZSI-UHFFFAOYSA-N |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Formamidinesulfinic Acid CAS 1758-73-2View Details
Formamidinesulfinic Acid CAS 1758-73-2View Details
1758-73-2 -
 Aminoiminomethanesulfinic acid CAS 1758-73-2View Details
Aminoiminomethanesulfinic acid CAS 1758-73-2View Details
1758-73-2 -
 Formamidinesulfinic acid CAS 1758-73-2View Details
Formamidinesulfinic acid CAS 1758-73-2View Details
1758-73-2 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
