Tefluthrin
- CAS NO.:79538-32-2
- Empirical Formula: C17H14ClF7O2
- Molecular Weight: 418.73
- MDL number: MFCD00274607
- EINECS: 616-699-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:26:02
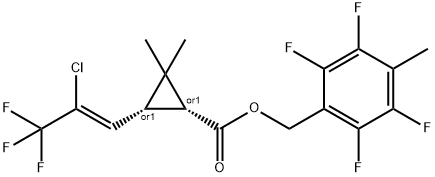
What is Tefluthrin?
Chemical properties
Pale Yellow Low Melting Solid
The Uses of Tefluthrin
Tefluthrin is used as a soil insecticide to control Coleoptera, Lepidoptera and Diptera in maize, sugar beet and other crops.
The Uses of Tefluthrin
Tefluthrin is a pyrethroid based insecticide. Tefluthrin is used in the control of a broad range of soil pests, including members of the Coleoptera, Lepidoptera, and Diptera.
Definition
ChEBI: A cyclopropanecarboxylate ester resulting from the formal condensation of the carboxy group of 3-[(1Z)-2-chloro-3,3,3-trifluoroprop-1-en-1-yl]-2,2-dimethylcyclopropanecarboxylic acid with the hydroxy group of 2,3,5,6-tetrafluoro-4-methylbe zyl alcohol.
Agricultural Uses
Insecticide, Miticide: A U.S. EPA restricted use Pesticide (RUP). Registered in the U.S. for use on corn. Elsewhere it is registered for use on a variety of crops, e.g., peanuts, sweet potato, sugarcane, cabbage, radish, Brussels sprouts, and strawberries. Registered for use in EU countries.
Trade name
FORCE®; FORCE® ST; FORZA®; JF 6064®; KOMET-RP®; PP 993®; R 151993®
Pharmacology
The commercialized compound, tefluthrin, was recommended to control soil-inhabiting pests (28). The most salient feature of the compound, which justifies its use exclusively for better performance of tefluthrin as a soil insecticide compared with cyhalothrin, is its approximately four orders ofmagnitude higher vapor pressure (V.P. = 8.4 vs. 0.001 mPa; see Table 6). Beside the quick insecticidal action attributed to fast penetration, tefluthrin also displays toxic symptoms at its sublethal dose, which could play an important role in field performance (179). The most active configuration of tefluthrin, (Z)(1RS)-cis-isomer mixture, is the optically active (Z)(1R)-cis-isomer.
Metabolic pathway
When a goat is dosed with 14C-tefluthrin orally, extensive metabolism occurs in the goat by ester cleavage and oxidation at a variety of positions on the molecules. Low radioactive residues are detected in the milk, fat, and muscle, with tefluthrin as the largest individual component of the residue. Higher residues are present in the kidney and liver, and only a small percentage of this residue is due to tefluthrin. The remainder in the kidney and liver is a complex mixture of metabolites. In total, eight metabolites are identified in the feces and urine as well as other tissues such as fat, muscle, kidney, and liver.
Degradation
Tefluthrin is a stable compound, being resistant to hydrolysis at pH 5-7 for at least 30 days. At pH 9, 7% hydrolysis occurred in 30 days. It is readily hydrolysed at higher pH to afford the cyclopropanecarboxylic acid (2) and 2,3,5,6-tetrafluoro-4-methylbenzyl alcohol (3). Exposure in aqueous solution at pH 7 to sunlight causes about 30% degradation in 31 days. Conversion to the trans-isomer occurred and hydrolysis to cis- and trans-2 and 3 followed.
Properties of Tefluthrin
| Melting point: | 44-46°C |
| Boiling point: | bp1.33hPa 156° |
| Density | 1.23 |
| vapor pressure | 8×10-3 Pa (20 °C) |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| Water Solubility | 0.02 mg l-1 (20 °C) |
| Merck | 13,9192 |
| BRN | 8398039 |
| EPA Substance Registry System | Tefluthrin (79538-32-2) |
Safety information for Tefluthrin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Tefluthrin
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran


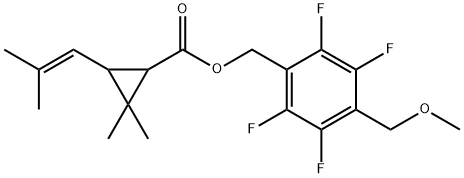
![[2,3,5,6-tetrafluoro-4-(methoxymethyl)phenyl]methyl 2,2-dimethyl-3-pro p-1-enyl-cyclopropane-1-carboxylate](https://img.chemicalbook.in/CAS/GIF/240494-70-6.gif)

You may like
-
 Tefluthrin 98% (HPLC) CAS 79538-32-2View Details
Tefluthrin 98% (HPLC) CAS 79538-32-2View Details
79538-32-2 -
 Tefluthrin CAS 79538-32-2View Details
Tefluthrin CAS 79538-32-2View Details
79538-32-2 -
 Tefluthrin CAS 79538-32-2View Details
Tefluthrin CAS 79538-32-2View Details
79538-32-2 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
