SULFENTRAZONE
- CAS NO.:122836-35-5
- Empirical Formula: C11H10Cl2F2N4O3S
- Molecular Weight: 387.19
- MDL number: MFCD00869633
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-27 08:46:37
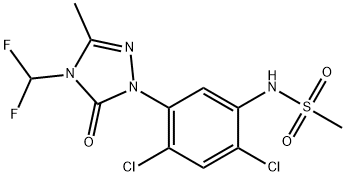
What is SULFENTRAZONE?
Description
Solubility. In water at 25 ?C: 100 mg/L at pH 6,
800 mg/mL at pH 7, 1,600 mg/mL at pH 7.5; soluble to
some extent in acetone and other polar organic solvents
Pka. 6.56
Stability. Stable
The Uses of SULFENTRAZONE
Sulfentrazone is an herbicide used for controlling sedges in turfgrass.
The Uses of SULFENTRAZONE
Herbicide.
Definition
ChEBI: Sulfentrazone is a member of the class of triazoles that is 5-oxo-1,2,4-triazole which is substituted at positions 1, 3, and 4 by 2,4-dichloro-5-[(methylsulfonyl)amino]phenyl, methyl, and difluoromethyl groups, respectively. A protoporphyrinogen oxidase inhibitor, it is used as a herbicide to control broad-leaved weeds in soya and tobacco crops. Not approved for use within the European Union. It has a role as an EC 1.3.3.4 (protoporphyrinogen oxidase) inhibitor, a herbicide and an agrochemical. It is a sulfonamide, a dichlorobenzene, an organofluorine compound and a member of triazoles.
Trade name
AUTHORITY® Sulfentrazone; CANOPY XL®; COVER®; F6285®; FMC® 97285; GAUNTLET®; SPARTAN®; SULFENTRAZONE® (F6285) 4F; SULFENTRAZONE® (F6285) 75DF
Metabolic pathway
When 14C-sulfentrazone is applied to coffee senna and sicklepod through the roots, 83% of the parent compound remains in coffee senna leaf tissue after 9 h exposure and in contrast, sicklepod takes up relatively less sulfentrazone through the root and metabolizes sulfentrazone in the foliage more rapidly than coffee senna. The primary detoxification reaction appears to be oxidation of the methyl group on the triazolinone ring, resulting in the formation of the more polar hydroxymethyl derivative. The aniline analog is identified as a plant-specific metabolite. The tolerance of sicklepod to sulfentrazone is primarily due to a relatively high rate of metabolism of sulfentrazone compared with coffee senna. When 14C-sulfentrazone is administered orally to rats, goats, and hens in a daily diet, administered radioactivity is quantitatively excreted in the urine, feces, or hen excreta. In all of the species, unchanged sulfentrazone and two non-conjugated metabolites are found, which are 3-hydroxymethyl and carboxylic acid derivatives, the latter of which decomposes at high temperature or acidic pH to give the corresponding desmethyl analog of sulfentrazone. In rats, a minor reduction metabolite is detected which is tentatively characterized as the 2,3-dihydro-3-hydroxymethyl derivative.
Properties of SULFENTRAZONE
| Melting point: | 75-78° |
| Boiling point: | 468.2±55.0 °C(Predicted) |
| Density | 1.21 g/cm3 |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 6.56(at 25℃) |
| form | Solid |
| color | Pale Brown to Beige |
| EPA Substance Registry System | Sulfentrazone (122836-35-5) |
Safety information for SULFENTRAZONE
Computed Descriptors for SULFENTRAZONE
New Products
Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Rifaximin EP Impurity-G N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
![2-(2,4-DICHLORO-PHENYL)-5-METHYL-2,4-DIHYDRO-[1,2,4]TRIAZOL-3-ONE](https://img.chemicalbook.in/CAS/GIF/79604-49-2.gif)

You may like
-
 122836-35-5 Sulfentrazone 99%View Details
122836-35-5 Sulfentrazone 99%View Details
122836-35-5 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
