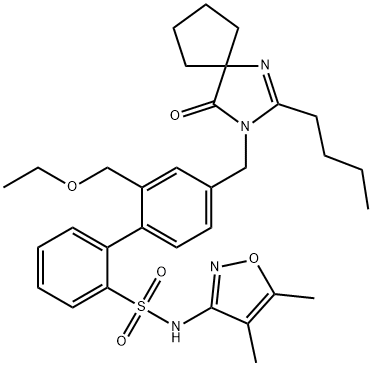
Sparsentan
Synonym(s):2-[4-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-2-(ethoxymethyl)phenyl]-N-(4,5-dimethyl-1,2-oxazol-3-yl)benzenesulfonamide;4′-[(2-Butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2′-(ethoxymethyl)[1,1′-biphenyl]-2-sulfonamide;BMS346567;BMS-346567;RE-021
- CAS NO.:254740-64-2
- Empirical Formula: C32H40N4O5S
- Molecular Weight: 592.75
- MDL number: MFCD16628036
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 19:14:41
What is Sparsentan ?
Absorption
Following administration of single doses of 200-1600 mg, the Cmax and AUC of sparsentan increase in a less than dose-proportional manner. Sparsentan has time-dependent pharmacokinetics, possibly due to it inducing its own metabolism over time, and at the approved recommended dosage, it reaches steady-state plasma levels within 7 days. Following a single oral dose of 400 mg, the Cmax, AUC and median time to peak plasma concentration of sparsentan are 6.97 μg/mL, 83 μg×h/mL, and 3 hours, respectively. Following daily doses of 400 mg sparsentan, the steady-state Cmax is 6.47 μg/mL, and the AUC is 63.6 μg×h/mL. The administration of a single oral dose (800 mg) of sparsentan with a high-fat, high-calorie meal (1000 kcal, 50% fat) increased the AUC and Cmax by 22% and 108%, respectively. With a single 200 mg dose, a high-fat, high-calorie meal did not have a clinically significant effect on sparsentan pharmacokinetics.
Toxicity
No overdose experience has been reported with sparsentan. Doses up to 1600 mg/day and 400 mg/day have been given to healthy volunteers and patients, respectively. Overdose of sparsentan may result in decreased blood pressure. Provide standard supportive measures, as required, in case of an overdose. Since sparsentan is highly protein-bound, dyalisis may not be effective. A 2-year rat carcinogenicity study where male and female mice received 0.7 and 26 times the AUC at the maximum recommended human dose (MRHD), respectively, found no evidence of increased incidence of neoplasia. A 26-week transgenic mouse study reported similar results. In vitro bacteria reverse mutation and chromosomal aberration assays and an in vivo rat micronucleus study did not find evidence of mutagenicity or clastogenicity for sparsentan. Sparsentan did not lead to an impairment of fertility in male or female rats and monkeys.
Description
Sparsentan (PS-433540, RE-021, DARA) is a dual endothelin type A receptor(ETA) and angiotensin II type 1 receptor antagonist.
The Uses of Sparsentan
RE 201 is used an Endothelin A receptor antagonist used in the treatment of hypertension.
Indications
Sparsentan is indicated to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression, generally a urine protein-to-creatinine ratio (UPCR) ≥1.5 g/g.
Background
Sparsentan is a dual antagonist of the endothelin type A receptor (ETAR) and the angiotensin II (Ang II) type 1 receptor (AT1R) with a similar affinity for both (9.3 nM for ETAR and 0.8 nM for AT1R). Sparsentan is first in its class and orally active, and was created by merging the structural elements of irbesartan, an AT1R antagonist, and biphenylsulfonamide, an ETAR antagonist.
In February 2023, the use of sparsentan to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk of rapid disease progression was approved by the FDA under accelerated approval based on reduction of proteinuria. Sparsentan was initially developed for the treatment of hypertension; however, it has shown to be efficient in the reduction of proteinuria in patients with IgAN and focal segmental glomerulosclerosis (FSGS). Compared to irbesartan, sparsentan reduces proteinuria to a greater extent. Furthermore, it is the first non-immunosuppressive therapy for the reduction of proteinuria in IgAN. The use of sparsentan may cause hepatotoxicity and embryo-fetal toxicity.
Definition
Sparsentan (RE-021) is a selective dual-acting receptor antagonist with affinity for endothelin (A type) and angiotensin II receptors (Type 1). Sparsentan combines the active moieties of the selective AT1 receptor antagonist irbesartan and a selective ETA receptor antagonist.
Biological Activity
Sparsentan (RE-021) is a highly potent dual angiotensin II and endothelin A receptor antagonist with Kis of 0.8 and 9.3 nM, respectively.
Pharmacokinetics
Sparsentan is a dual endothelin and angiotensin II receptor antagonist. At week 36, the exposure-response (E-R) relationship between sparsentan exposure and the percentage reduction from baseline in urine protein-to-creatinine ratio (UPCR) was not statistically significant over the observed sparsentan exposure range. E-R relationships were not statistically significant for any grade of hypotension or the worst grade of peripheral edema.
In healthy subjects, sparsentan caused QTcF prolongation with a maximal mean effect of 8.8 msec at 800 mg and 8.1 msec at 1600 mg. The mechanism behind the observed QTc prolongation is unknown but is unlikely to be mediated via direct inhibition of hERG channels. At the recommended dose, no clinically relevant QTc prolongation is expected. The use of sparsentan may cause hepatotoxicity, embryo-fetal toxicity, hypotension, acute kidney injury, hyperkalemia, and fluid retention.
in vitro
Sparsentan dose dependently antagonizes the angiotensin II-induced pressor response with an ED50 value of 0.8 μmol/kg iv and 3.6 μmol/kg po. Sparsentan also shows efficacious and long acting in the big ET-1-induced pressor model. Sparsentan causes a significant lowering of blood pressure at the lowest dose tested (10 μmol/kg/day) in spontaneously hypertensive rats. Sparsentan shows good oral bioavailability in rats, dogs, and monkeys, averaging 40%, 86%, and 21% F, respectively. At 100 μmol/kg/day, Sparsentan reduces the blood pressure from 170 to less than 100 mmHg during the course of the drug’s pharmacokinetic duration. Sparsentan at 100 μmol/kg/day essentially converts the spontaneously hypertensive rats into normotensive rats during the course of its pharmacokinetic duration.
Metabolism
Sparsentan is mainly metabolized by cytochrome P450 3A.
Properties of Sparsentan
| Melting point: | 148 °C(Solv: isopropanol (67-63-0); water (7732-18-5)) |
| Boiling point: | 744.4±70.0 °C(Predicted) |
| Density | 1.28±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO: soluble |
| form | A crystalline solid |
| pka | 7.06±0.50(Predicted) |
| color | White to off-white |
Safety information for Sparsentan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Sparsentan
| InChIKey | WRFHGDPIDHPWIQ-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=C(CN3C(=O)C4(CCCC4)N=C3CCCC)C=C2COCC)=CC=CC=C1S(NC1C(C)=C(C)ON=1)(=O)=O |
Sparsentan manufacturer
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 254740-64-2 98+View Details
254740-64-2 98+View Details
254740-64-2 -
 Sparsentan CAS 254740-64-2View Details
Sparsentan CAS 254740-64-2View Details
254740-64-2 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8