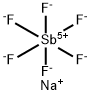
Sodium hexafluoroantimonate
- CAS NO.:16925-25-0
- Empirical Formula: F6NaSb
- Molecular Weight: 258.74
- MDL number: MFCD00003482
- EINECS: 240-989-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:13:03
What is Sodium hexafluoroantimonate?
Chemical properties
WHITE TO OFF-WHITE FINE CRYSTALLINE POWDER
Properties of Sodium hexafluoroantimonate
| Density | 3.375 g/mL at 25 °C (lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: slightly soluble(lit.) |
| form | Powder |
| color | White to off-white |
| Specific Gravity | 3.375 |
| Water Solubility | SOLUBLE |
| Sensitive | Air Sensitive |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| InChI | InChI=1S/6FH.Na.Sb/h6*1H;;/q;;;;;;+1;+5/p-6 |
| CAS DataBase Reference | 16925-25-0(CAS DataBase Reference) |
| EPA Substance Registry System | Antimonate(1-), hexafluoro-, sodium, (OC-6-11)- (16925-25-0) |
Safety information for Sodium hexafluoroantimonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium hexafluoroantimonate
| InChIKey | HKLMYZVMEYYVBS-UHFFFAOYSA-H |
| SMILES | [Sb+5]([F-])([F-])([F-])([F-])([F-])[F-].[Na+] |
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 Sodium hexafluoroantimonate(V) CAS 16925-25-0View Details
Sodium hexafluoroantimonate(V) CAS 16925-25-0View Details
16925-25-0 -
 Sodium hexafluoroantimonate(V) CAS 16925-25-0View Details
Sodium hexafluoroantimonate(V) CAS 16925-25-0View Details
16925-25-0 -
 Sodium hexafluoroantimonate 99% CAS 16925-25-0View Details
Sodium hexafluoroantimonate 99% CAS 16925-25-0View Details
16925-25-0 -
 Sodium hexafluoroantimonate(V) CAS 16925-25-0View Details
Sodium hexafluoroantimonate(V) CAS 16925-25-0View Details
16925-25-0 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
