SMCC crosslinker
Synonym(s):N-Succinimidyl 4-(maleimidomethyl)cyclohexanecarboxylate;SMCC;Succinimidyl- trans-4-(N-maleimidylmethyl)cyclohexane-1-carboxylate;Succinimidyl-trans-4-(N-maleimidylmethyl)cyclohexane-1-carboxylate
- CAS NO.:64987-85-5
- Empirical Formula: C16H18N2O6
- Molecular Weight: 334.32
- MDL number: MFCD00009634
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:22:19
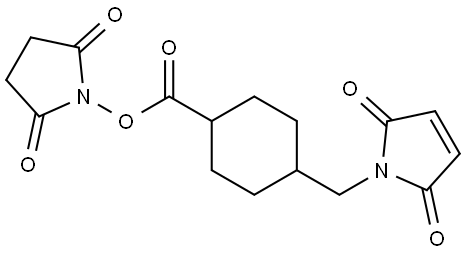
What is SMCC crosslinker?
Description
SMCC is a hetero-bifunctional crosslinker that contain N-hydroxysuccinimide (NHS) ester and maleimide groups that allow covalent conjugation of amine- and sulfhydryl-containing molecules.
Chemical properties
White Crystalline Solid
The Uses of SMCC crosslinker
A sulfhydryl and amino reactive heterobifunctional protein crosslinking reagent. It conjugates anti-digoxin F(ab’)2 fragments to ?-galactosidase. Conjugates hIgG to alkaline phosphatase. Spacer Arm: 11.6 Angstroms
The Uses of SMCC crosslinker
A sulfhydryl and amino reactive heterobifunctional protein crosslinking reagent. It conjugates anti-digoxin F(abTaIII)2 fragments to l-B-galactosidase. Conjugates hIgG to alkaline phosphatase.Spacer Arm: 11.6 Angstroms.
What are the applications of Application
SMCC is a heterobifunctional sulfhydryl- and amine- reactive crosslinker, 11.6 Angstrom spacer arm
Definition
ChEBI: Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate is an N-hydroxysuccinimide ester derived from 4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid. It is a member of maleimides and a N-hydroxysuccinimide ester.
General Description
4-(N-Maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester (SMCC) is a heterobifunctional cross-linking reagent incorporating an extended spacer with amine and sulfhydryl reactivity. It is typically coupled initially to molecules containing primary amine by amide bond buffered at pH 7.5 (6.5-8.5). The second coupling is specific for molecules containing free sulfhydryl by thioether linkage buffered at pH 6.8 (6.5-7.0). SMCC contains nine atom linker. An extended aliphatic spacer stabilizes the maleimide prior to coupling compared to aromatic spacers.
Chemical Reactivity
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) is a non-cleavable and membrane permeable crosslinker. It contains an amine-reactive N -hydroxysuccinimide (NHS ester) and a sulfhydryl-reactive maleimide group. NHS esters react with primary amines at pH 7-9 to form stable amide bonds. Maleimides react with sulfhydryl groups at pH 6.5-7.5 to form stable thioether bonds. The maleimide groups of SMCC and Sulfo-SMCC and are unusually stable up to pH 7.5 because of the cyclohexane bridge in the spacer arm.
Properties of SMCC crosslinker
| Melting point: | 180-182 °C (lit.) |
| Boiling point: | 501.7±42.0 °C(Predicted) |
| Density | 1.42±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | chloroform: 50 mg/mL |
| form | powder |
| pka | -2.24±0.20(Predicted) |
| color | White to Off-White |
| Sensitive | Moisture & Light Sensitive |
| BRN | 1555271 |
| InChI | InChI=1S/C16H18N2O6/c19-12-5-6-13(20)17(12)9-10-1-3-11(4-2-10)16(23)24-18-14(21)7-8-15(18)22/h5-6,10-11H,1-4,7-9H2 |
| CAS DataBase Reference | 64987-85-5(CAS DataBase Reference) |
Safety information for SMCC crosslinker
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for SMCC crosslinker
| InChIKey | JJAHTWIKCUJRDK-UHFFFAOYSA-N |
| SMILES | C1(C(ON2C(=O)CCC2=O)=O)CCC(CN2C(=O)C=CC2=O)CC1 |
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
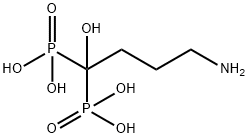
![Phosphonic acid, P,P'-[4-[[[4-[(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)methyl]cyclohexyl]carbonyl]amino]-1-hydroxybutylidene]bis-](https://img.chemicalbook.in/CAS/20210111/GIF/1426523-52-5.gif)


You may like
-
 N-Succinimidyl 4-(N-Maleimidomethyl)cyclohexanecarboxylate CAS 64987-85-5View Details
N-Succinimidyl 4-(N-Maleimidomethyl)cyclohexanecarboxylate CAS 64987-85-5View Details
64987-85-5 -
 Succinimidyl 4-(N-maleimidomethyl)cyclohexanecarboxylate 98% CAS 64987-85-5View Details
Succinimidyl 4-(N-maleimidomethyl)cyclohexanecarboxylate 98% CAS 64987-85-5View Details
64987-85-5 -
 4-(N-Maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester CAS 64987-85-5View Details
4-(N-Maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester CAS 64987-85-5View Details
64987-85-5 -
 SMCC CAS 64987-85-5View Details
SMCC CAS 64987-85-5View Details
64987-85-5 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
