satratoxin G
- CAS NO.:53126-63-9
- Empirical Formula: C29H36O10
- Molecular Weight: 544.59
- MDL number: MFCD01684361
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-17 18:22:24
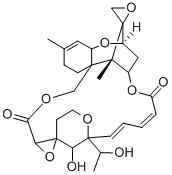
What is satratoxin G?
Biological Activity
Satratoxin G is a macrocyclic trichothecene mycotoxin that has been found in S. chartarum.1,2 It induces cleavage of caspase-3 and poly(ADP-ribose) polymerase (PARP) in HL-60 cells when used at a concentration of 40 nM and is cytotoxic to HepG2, Hep-2, Caco-2, A204, U937, and Jurkat cells (IC50s = 2.2-9.7 ng/ml).3,4 Intranasal administration of satratoxin G (500 μg/kg) induces apoptosis of olfactory sensory neurons in olfactory epithelium and ethmoid turbinate expression of the genes encoding IL-1α, IL-1β, IL-6, TNF-α, and MIP-2 in mice.2 Satratoxin G induces lethality in 4 week-old male mice (LD50 = 1.23 mg/kg, i.p.).1
References
|1. Yoshizawa, T., Ohtsubo, K., Sasaki, T., et al. Acute toxicities of satratoxins G and H in mice--a histopathological observation with special reference to the liver injury caused by satratoxin G. Proc. Jpn. Assoc. Mycotoxicol. 23, 53-57 (1986).|2. Islam, Z., Harkema, J.R., and Pestka, J.J. Satratoxin G from the black mold Stachybotrys chartarum evokes olfactory sensory neuron loss and inflammation in the murine nose and brain. Environ. Health Perspect. 114(7), 1099-1107 (2006).|3. Nagase, M., Shiota, T., Tsushima, A., et al. Molecular mechanism of satratoxin-induced apoptosis in HL-60 cells: Activation of caspase-8 and caspase-9 is involved in activation of caspase-3. Immunol. Lett. 84(1), 23-27 (2002).|4. Nielsen, C., Casteel, M., Didier, A., et al. Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Res. 25(2), 77-84 (2009).
Properties of satratoxin G
| storage temp. | Store at -20°C |
| solubility | Dichloromethane: soluble,DMSO: soluble,Ethanol: soluble |
| form | A crystalline solid |
Safety information for satratoxin G
Computed Descriptors for satratoxin G
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![1,6-Dioxaspiro[2.5]octane](https://img.chemicalbook.in/CAS/GIF/185-72-8.gif)
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
