Protoporphyrin IX
Synonym(s):3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphinedipropionic acid;8,13-Divinyl-3,7,12,17-tetramethyl-21H,23H-porphine-2,18-dipropionic acid zinc derivative;Kammerer’s porphyrin;Ooporphyrin;Protoporphyrin IX zinc(II) complex
- CAS NO.:553-12-8
- Empirical Formula: C34H34N4O4
- Molecular Weight: 562.66
- MDL number: MFCD00151109
- EINECS: 209-033-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-22 22:14:22
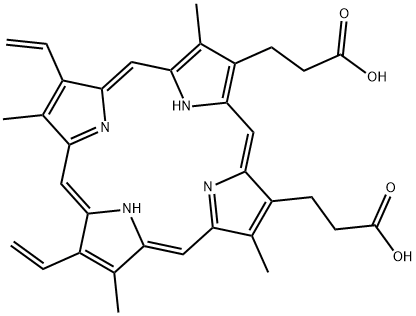
What is Protoporphyrin IX?
The Uses of Protoporphyrin IX
Protoporphyrin IX has been used:
- as a standard in protoporphyrin assays
- in fluorescence spectra analysis
- to treat cells in cell culture to study heme-mediated ferroportin 1 transcription
The Uses of Protoporphyrin IX
hepatoprotectant
What are the applications of Application
Protoporphyrin-9 is the key porphyrin precursor to heme sites and chlorophyll in animal and plant cells
Definition
ChEBI: A cyclic tetrapyrrole that consists of porphyrin bearing four methyl substituents at positions 3, 8, 13 and 17, two vinyl substituents at positions 7 and 12 and two 2-carboxyethyl substituents at positions 2 and 18. The parent of the class of protoporphyri s.
General Description
Protoporphyrin IX belongs to the porphyrin family, which are a class of tetrapyrroles. It is the iron-free form of hemin and amphiphilic in nature. It is the precursor of heme in its biosynthetic pathway.
Biochem/physiol Actions
Protoporphyrin IX?levels are elevated in?tumor?cells due to?metabolism anomalies?compared to normal?cells.
Enzyme inhibitor
This iiron-free, immediate precusor of heme (FWfree-acid = 562.67 g/mol; CAS 553-12-8; brownish-yellow solid; soluble in a number of organic solvents; disodium and dipotassium salts solublized in the presence of Tween 80) has lmax values in 25% HCl are 602.4, 582.2, and 557.2 nm. Protoporphryrin IX is also an activator of guanylate cyclase. See also Iron Protoporphyrin IX; Heme; Hemin Target(s): aminolevulinate aminotransferase; 5-aminolevulinate synthase; glutamate: glyoxylate aminotransferase; glutathione S-transferase; glyoxalase I, or lactoylglutathione lyase; guanylate cyclase; heme oxygenase; hydroxymethylbilane synthase, or porphobilinogen deaminase; nitric-oxide synthase; porphobilinogen synthase, or 5-aminolevulinate dehydratase; succinyl-CoA synthetase; tryptophan pyrrolase, or tryptophan 2,3-dioxygenase; and uroporphyrinogen decarboxylase.
Purification Methods
Protoporphyrin IX (3,18-divinyl-2,7,13,17-tetramethylporphin-8,12-dipropionic acid, ooporphyrin) [553-12-8] M 562.7, pKEst ~ 4.8. Protoporphyrin IX is purified by dissolving (4g) in 98-100% HCOOH (85mL), diluting with dry Et2O (700mL) and keeping at 0o overnight. The precipitate is collected and washed with Et2O, then H2O, and dried in a vacuum at 50o over P2O5. It crystallises from aqueous pyridine and from Et2O in monoclinic, brownish-yellow prisms. The UV max values in 25% HCl are 557.2, 582.2 and 602.4nm. It is freely soluble in ethanolic HCl, AcOH, CHCl3, and Et2O containing AcOH. It forms sparingly soluble diNa and diK salts. [Ramsey Biochemical Preparations 3 39 1953, UV: Holden Aust J. Exptl Biol and Med Sci 15 412 1937, Garnick J Biol Chem 175 333 1948, IR: Falk & Willis Aust J Sci Res [A] 4 579 1951, Beilstein 26 IV 3042.]
Toxicity evaluation
protoporphyrin IX, is highly toxic in the presence of light and molecular oxygen, killing photosynthetic plants very quickly through the generation of singlet oxygen.
Properties of Protoporphyrin IX
| Melting point: | >300 °C(Solv: acetone (67-64-1)) |
| Boiling point: | 629.06°C (rough estimate) |
| Density | 1.1829 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly) |
| form | Powder |
| color | purple |
| Water Solubility | 106.9mg/L(25 ºC) |
| Sensitive | light sensitive |
| Merck | 13,7990 |
| BRN | 380795 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 553-12-8 |
| NIST Chemistry Reference | Protoporphyrin-1x(553-12-8) |
| EPA Substance Registry System | Protoporphyrin IX (553-12-8) |
Safety information for Protoporphyrin IX
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Protoporphyrin IX
| InChIKey | FEDYMSUPMFCVOD-UJJXFSCMSA-N |
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran


You may like
-
 Protoporphyrin IX, ≥95% CAS 553-12-8View Details
Protoporphyrin IX, ≥95% CAS 553-12-8View Details
553-12-8 -
 Protoporphyrin IX 95% (HPLC) CAS 553-12-8View Details
Protoporphyrin IX 95% (HPLC) CAS 553-12-8View Details
553-12-8 -
 Protoporphyrin IX CAS 553-12-8View Details
Protoporphyrin IX CAS 553-12-8View Details
553-12-8 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
