Procarbazine hydrochloride
Synonym(s):N-(1-Methylethyl)-4-[(2-methylhydrazinyl)methyl]benzamide hydrochloride
- CAS NO.:366-70-1
- Empirical Formula: C12H20ClN3O
- Molecular Weight: 257.76
- MDL number: MFCD00072082
- EINECS: 206-678-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-24 18:36:04
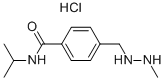
What is Procarbazine hydrochloride ?
Chemical properties
Crystalline Solid
Chemical properties
Procarbazine is a white to pale yellow crystal- line powder with a slight odor.
The Uses of Procarbazine hydrochloride
Procarbazine hydrochloride USP is used to traet Hodgkin’s disease; non-Hodgkin’s lymphomas; lung cancer.
The Uses of Procarbazine hydrochloride
Antineoplastic;DNA replication inhibitor
The Uses of Procarbazine hydrochloride
Antineoplastic.
What are the applications of Application
Procarbazine Hydrochloride is an antineoplastic agent
Definition
ChEBI: A hydrochloride obtained by combining procarbazine with one equivalent of hydrochloric acid. An antineoplastic chemotherapy drug used for treatment of Hodgkin's lymphoma. Metabolism yields azo-procarbazine and hydrogen peroxide, which results in the breaki g of DNA strands.
brand name
Matulane (Sigma-Tau).
General Description
White to pale yellow crystalline powder with a slight odor. Acid to litmus.
General Description
Procarbazine is available in 50-mg tablets for oral administrationin the treatment of Hodgkin’s (part of MOPP) andnon-Hodgkin’s disease, brain cancer, and mycosis fungoides.The major mechanisms of resistance appear to beenhanced activity of DNA-repair enzymes including enhancedO-6-alkylguanine DNA transferase (AGAT),which removes the methyl group from the O-6 of guanine.The agent is rapidly and completely absorbed after oral administrationand extensively metabolized in the liver togive azo-procarbazine followed by further oxidation tomethyldiazine and the aldehyde. The parent drug andmetabolites cross the blood-brain barrier. Elimination occursin the urine mostly as metabolites with an eliminationhalf-life of 1 hour. Myelosuppression is dose limiting, generallypresenting as thrombocytopenia that may be followedby leucopenia. Glucose-6-phosphate dehydrogenasedeficient patients may develop hemolytic anemia duringprocarbazine therapy. Other adverse effects include nausea,vomiting, hypersensitivity, flulike symptoms, amenorrhea,and azoospermia. Central nervous symptom effectsmay be seen, including lethargy, confusion, neuropathies,and seizure.
Air & Water Reactions
In the presence of moisture or in aqueous solution undergoes oxidation by atmospheric oxygen. Water soluble.
Reactivity Profile
Procarbazine hydrochloride is very sensitive to light. Stability is highest in aqueous acid and decreases with increasing pH. Degrades rapidly in alcoholic media and more slowly in aqueous media .
Fire Hazard
Flash point data for Procarbazine hydrochloride are not available; however, Procarbazine hydrochloride is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. Poison by an unspecified route. Moderately toxic by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and HCl. Used as a chemotherapeutic agent.
Potential Exposure
Procarbazine is available in capsule form. The primary use of this drug is as an antineoplastic agent in the treatment of advanced Hodgkin’s disease, and oat-cell carcinoma of the lung. The hydrochloride com- pound is used in treatment. The FDA approved use of pro- carbazine hydrochloride in 1969 and indicated that the drug should be used as an adjunct to standard therapy. Possible exposure occurs during manufacture of the drug and direct exposure during its subsequent administration to patients. Some of the metabolites of procarbazine hydrochloride are both carcinostatic and carcinogenic.
Veterinary Drugs and Treatments
In veterinary medicine, procarbazine is used as part of MOPP protocols (mechlorethamine, vincristine, procarbazine, prednisone) to treat lymphomas in dogs and cats. It may be of benefit in treating granulomatous meningoencephalitis (GME) in dogs.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quanti- ties shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Properties of Procarbazine hydrochloride
| Melting point: | 223°C |
| Density | 8.3 g/cm3 |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO: ≥18mg/mL |
| form | powder |
| color | white to tan |
| Water Solubility | >=10 g/100 mL at 21.5 ºC |
| Merck | 14,7758 |
| CAS DataBase Reference | 366-70-1(CAS DataBase Reference) |
| IARC | 2A (Vol. 26, Sup 7) 1987 |
| EPA Substance Registry System | Procarbazine hydrochloride (366-70-1) |
Safety information for Procarbazine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H341:Germ cell mutagenicity H350:Carcinogenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Procarbazine hydrochloride
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran

![Isopropyl-α-[2-methylhydrazino]-p-toluamide](https://img.chemicalbook.in/CAS/GIF/671-16-9.gif)
You may like
-
 366-70-1 Procarbazine hydrochloride 98%View Details
366-70-1 Procarbazine hydrochloride 98%View Details
366-70-1 -
 Procarbazine Hydrochloride CAS 366-70-1View Details
Procarbazine Hydrochloride CAS 366-70-1View Details
366-70-1 -
 Procarbazine hydrochloride CAS 366-70-1View Details
Procarbazine hydrochloride CAS 366-70-1View Details
366-70-1 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
