PREMARIN
- CAS NO.:481-97-0
- Empirical Formula: C18H22O5S
- Molecular Weight: 350.43
- MDL number: MFCD00867391
- EINECS: 207-575-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-24 17:36:03
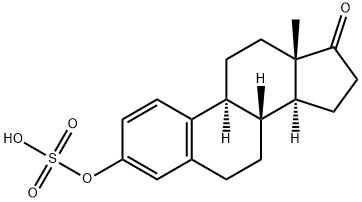
What is PREMARIN?
Absorption
Estropipate is well absorbed through the skin and gastrointestinal tract. When applied for a local action, absorption is usually sufficient to cause systemic effects.
The Uses of PREMARIN
Estrone Sulfate is a precursor of estrogen and is a circulating steroid found in men, non-pregnant women, and post-menopausal women.
Background
Estrone sulfate (as estropipate) is a form of estrogen. It has several uses such as: alleviate symptoms of menopause as hormone replacement therapy, treatment some types of infertility, treatment of some conditions leading to underdevelopment of female sexual characteristics, treatment of vaginal atrophy, treatment of some types of breast cancer (particularly in men and postmenopausal women), treatment of prostate cancer and prevention of osteoporosis.
Indications
Estropipate is used for the treatment of moderate to severe vasomotor symptoms associated with the monopause, and moderate to severe symptoms of vulval and vaginal atrophy associated with the menopause. It is also used to treat hypoestrogenism due to hypogonadism, castration or primary ovarian failure, and prevent postmenopausal osteoporosis.
Definition
ChEBI: A steroid sulfate that is the 3-sulfate of estrone.
Pharmacokinetics
Estropipate is an estrogenic substance. It acts as naturally produced estrogen does. Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Properties of PREMARIN
| Melting point: | 210 °C (decomp) |
| Density | 1.349±0.06 g/cm3(Predicted) |
| pka | -3.84±0.18(Predicted) |
| EPA Substance Registry System | Estra-1,3,5(10)-trien-17-one, 3-(sulfooxy)- (481-97-0) |
Safety information for PREMARIN
Computed Descriptors for PREMARIN
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
![1,3,5[10]-ESTRATRIEN-3-OL-17-ONE SULFATE POTASSIUM SALT](https://img.chemicalbook.in/CAS/GIF/1240-04-6.gif)
![ESTRONE SULFATE AMMONIUM SALT, [6,7-3H(N)]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB2431051.gif)


You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
