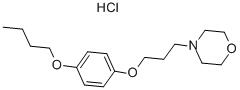
pramocaine
- CAS NO.:140-65-8
- Empirical Formula: C17H27NO3
- Molecular Weight: 293.4
- MDL number: MFCD00864456
- EINECS: 205-425-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
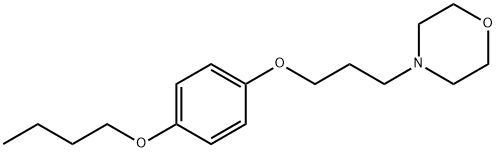
What is pramocaine?
Absorption
There is minimal absorption after topical administration and it is not given orally.
Toxicity
LD50 Mouse ip 300 mg/kg, LD50 Mouse sc 750 mg/kg
Originator
Tronothane, Abbott, US ,1954
The Uses of pramocaine
Anesthetic (topical).
Indications
It is indicated for temporary relief of pain and pruritus from minor lip and skin irritations as well as for temporary relief from pain, burning, itching and discomfort associated with hemorrhoids and other anorectal/anogenital disorders.
Background
Pramocaine (also known as pramoxine or pramoxine HCI) is a topical anesthetic and antipruritic. It is used for many dermatological and anorectal/anogenital conditions including minor cuts/burns, insect bites, hives/rashes due to poison ivy exposure, hemorrhoids and other anorectal/anogenital disorders. Pramocaine is available by itself and in combination with other medications in various topical preparations. It works by preventing ionic fluctuations needed for neuron membrane depolarization and action potential propagation.
Definition
ChEBI: A member of the class of morpholines that is morpholine substituted at the nitrogen atom by a 3-(4-butoxyphenoxy)propyl group.
Manufacturing Process
About 5.6 g of potassium hydroxide is dissolved in about 150 cc of refluxing ethanol, and then about 16.6 g of hydroquinone monobutyl ether is added to the alcoholic solution. When the hydroquinone is dissolved, about 16.3 g of γmorpholinopropyl chloride (dissolved in a small amount of ethanol) is added to the refluxing solution. The solution is refluxed for about 24 hours and then cooled. The product is recovered by filtering the reaction mixture and then removing the solvent by vacuum distillation. The oily residue is acidified and shaken with ether. The acidic phase is made strongly alkaline with 40% sodium hydroxide, and the oil which separates is extracted into ether. The ethereal phase is dried, and the solvent removed by vacuum distillation. The product distills at 183° to 184°C at a pressure of 2.8 mm. The hydrochloride salt of the foregoing base is prepared by dissolving the base in ether and acidifying with hydrochloric acid and is found to have a MP of 181° to 183°C.
brand name
Tronolane (Ross); Tronothane (Abbott).
Therapeutic Function
Local anesthetic
Pharmacokinetics
Pramocaine temporarily relieves pain, pruritis, burning and discomfort associated with minor lip and skin irritations and hemorrhoid's by inhibiting voltage gated sodium channels on neurons.
Metabolism
Not Available
Properties of pramocaine
| Boiling point: | bp6 196°; bp2.8 183-184° |
| Density | 1.0112 (rough estimate) |
| refractive index | 1.5420 (estimate) |
| pka | 7.18±0.10(Predicted) |
| Water Solubility | 3.574mg/L(22.5 ºC) |
| CAS DataBase Reference | 140-65-8 |
Safety information for pramocaine
Computed Descriptors for pramocaine
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 140-65-8 Pramoxine 98%View Details
140-65-8 Pramoxine 98%View Details
140-65-8 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5