Potassium carbonate
Synonym(s):Dipotassium carbonate;potash;Potassium carbonate
- CAS NO.:584-08-7
- Empirical Formula: K2CO3
- Molecular Weight: 138.21
- MDL number: MFCD00011382
- EINECS: 209-529-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:20:01
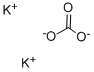
What is Potassium carbonate?
Description
Potassium carbonate(K2CO3) is a white, crystalline, salt that forms basic aqueous solutions used in the production of fertilizer, glass, ceramics, explosives, soaps, chemicals, and wool treatments. It was the main compound once referred to as potash, although the term today is not reserved exclusively for potassium carbonate, but for several potassium salts. In the fertilizer industry, potash refers to potassium oxide, K2O, rather than potassium carbonate.
Chemical properties
A white salt, soluble in water (insoluble in ethanol) which forms a strongly alkaline solution. Potassium carbonate can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It presents a large capacity to absorb moisture.Corrosive to metals and tissue. Density 12.8 lb /gal. It has low solubility in ethanol, acetone, and many other common organic solvents. Used to make soaps, other potassium compounds, in liquid fertilizers.
The Uses of Potassium carbonate
Potassium carbonate is a common flux combined with titanium dioxide to produce frits used in ceramics. Potassium carbonate is used in the chemical industry as a source of inorganic potassium salts (potassium silicates, potassium bicarbonate), which are used in fertilizers, soaps, adhesives, dehydrating agents, dyes, and pharmaceuticals. Potassium carbonate used to make potassium lye produces soft soaps, which are liquids or semisolids rather than solids. Other uses of potassium carbonate includes use as a fire suppressant in extinguishers, as a CO2 absorbent for chemical processes and pollution control, an antioxidant in rubber additives, and in pharmaceutical formulations.
Production Methods
Potassium carbonate can be obtained from ash or by electrolysis of potassium chloride. Another method involves reacting potassium hydroxide with carbon dioxide. The resulting liquid carbonate contains approximately 50% potassium carbonate in water. However, the solid product, which contains over 70% potassium carbonate, is expensive and only suitable for certain types of acidic soil. Additionally, neutralizing caustic potash with carbon dioxide also yields potassium carbonate.
Agricultural Uses
Potassium carbonate is used in agriculture and food production. Potassium carbonate is used as a spray or drip fertilizer and also as a constituent of compound fertilizers. Its high water solubility and alkaline property make it useful for supplying potassium to acidic soils, especially in vineyards and orchards. It is used to produce food additives like potassium sorbate and monopotassium phosphate.
Industrial uses
Potassium carbonate is used for numerous applications. Its primary use is in the production of specialty glasses and ceramics. It is used to make optical glass, glass used for video screens in televisions and computers, and laboratory glassware.
Potassium carbonate is a common flux combined with titanium dioxide to produce frits used in ceramics. A frit is a calcined mixture of fine silica, a pigment, and a flux that is ground a specific particle size and used to produce glazes, enamels, and additives in glass making.
Safety Profile
Poison by ingestion. A strong caustic. Incompatible with KCO, chlorine trifluoride, magnesium. Mutation data reported. When heated to decomposition, potassium carbonate emits toxic fumes of K2O.
Properties of Potassium carbonate
| Melting point: | 891 °C (lit.) |
| Boiling point: | decomposes [STR93] |
| Density | 2.43 g/mL at 25 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| appearance | White solid |
| form | powder |
| pka | 10.33[at 20 ℃] |
| color | Yellow |
| Specific Gravity | 2.29 |
| Odor | at 100.00?%. odorless |
| PH | 10.52(1 mM solution);11(10 mM solution);11.36(100 mM solution); |
| Water Solubility | 1120 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,7619 |
| BRN | 4267587 |
| Dielectric constant | 5.6(16.0℃) |
| Stability: | Stable. Incompatible with moisture, acids, magnesium bromine trifluoride and magnesium bromine trichloride. |
| CAS DataBase Reference | 584-08-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Dipotassium carbonate(584-08-7) |
| EPA Substance Registry System | Potassium carbonate (584-08-7) |
Safety information for Potassium carbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium carbonate
Potassium carbonate manufacturer
JSK Chemicals
New Products
N,O-Dimethylhydroxylamine hydrochloride DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide 3-Hydroxypropionitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Potassium carbonate 98%View Details
Potassium carbonate 98%View Details -
 POTASSIUM CARBONATE (POWDER) 99%View Details
POTASSIUM CARBONATE (POWDER) 99%View Details -
 Potassium Carbonate 99%View Details
Potassium Carbonate 99%View Details -
 Potassium carbonate 98%View Details
Potassium carbonate 98%View Details -
 Potassium carbonate CAS 584-08-7View Details
Potassium carbonate CAS 584-08-7View Details
584-08-7 -
 Potassium carbonate CAS 584-08-7View Details
Potassium carbonate CAS 584-08-7View Details
584-08-7 -
 Potassium carbonate CAS 584-08-7View Details
Potassium carbonate CAS 584-08-7View Details
584-08-7 -
 Potassium carbonate CASView Details
Potassium carbonate CASView Details
