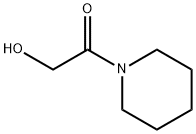
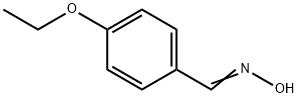
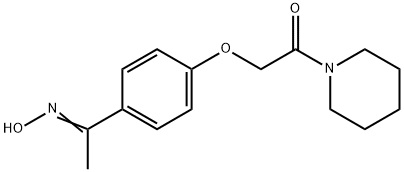
Pifoxime
- CAS NO.:31224-92-7
- Empirical Formula: C15H20N2O3
- Molecular Weight: 276.33
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 15:21:26
What is Pifoxime?
Originator
Flamanil ,Salvoxyl-Wander, France ,1975
Manufacturing Process
(A) Preparation of p-Acetylphenoxyacetic Acid: p-Hydroxy-acetophenone is treated with chloroacetic acid in aqueous solution in the presence of sodium hydroxide. The desired acid is then isolated from its sodium salt in a total yield of 80 to 82%, excess of p-hydroxy-acetophenone having been extracted with methylene chloride.
(B) Preparation of Methyl p-Acetylphenoxy-Acetate: A mixture of 80 g of the acid obtained in (A) and 200 ml of methyl alcohol in 600 ml of dichloromethane is refluxed in the presence of sulfuric acid. The desired ester is isolated in accordance with a method known per se, and recrystallized. When the refluxing period is 12 hours, the ester is obtained with a yield of 70%. When the refluxing period is 18 hours, the yield for this ester is 85%.
(C) Preparation of N-(p-Acetylphenoxy-Acetyl)-Piperidine: The ester from (B) is refluxed for 8 hours with 2.5 mols of thoroughly dried piperidine. Then 1 volume of water is added and the product is left to crystallize in the cold. The desired amide is obtained in an 80% yield.
(D) Preparation of N-(p-[1-Isonitrosoethyl]-Phenoxy-Acetyl)-Piperidine: The amide from (C) is refluxed for 5 hours with technical (98%) hydroxylamine and alcohol denatured with methanol. The desired product is obtained in a 75% yield.
In semiindustrial synthesis, to achieve better yields, it is possible to omit (A), by directly preparing the ester (B) by reaction of p-hydroxy acetophenone on ethyl 2-bromoacetate in the presence of potassium carbonate in butanone. The yield of ester is 90%, and elimination of excess of phydroxyacetophenone is effected by washing with sodium hydroxide.
Therapeutic Function
Antiinflammatory
Properties of Pifoxime
| Boiling point: | 494.7±30.0 °C(Predicted) |
| Density | 1.18±0.1 g/cm3(Predicted) |
| pka | 11.50±0.70(Predicted) |
Safety information for Pifoxime
Computed Descriptors for Pifoxime
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 4-Bromo Benzylcyanide 99%View Details
4-Bromo Benzylcyanide 99%View Details
26451-08-1 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5