Phenanthridine
Synonym(s):Benzo[c]quinoline
- CAS NO.:229-87-8
- Empirical Formula: C13H9N
- Molecular Weight: 179.22
- MDL number: MFCD00004989
- EINECS: 205-934-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:23:27
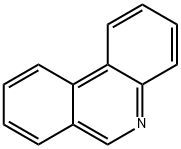
What is Phenanthridine?
Chemical properties
white to beige powder
History
Phenanthridine was first discovered by Ame Pictet and H. J. Ankersmit in 1891 by pyrolysis of the condensed product of benzaldehyde and aniline. Earlier, phenanathridine and related compounds were prepared using mainly Pictect-Hurbert and modified Morgan-Walls type of condensation reactions.
The Uses of Phenanthridine
Phenanthridine is an isomeric compound of acridine. It is a nitrogen heterocycle that is the basis of DNA-binding fluorescent dyes through intercalation. Ethidium bromide and propidium iodide are the examples of such intercalating dyes. It is used as a dye in biological and chemical sensors. It is also used in the study of ecotoxicity and rotational spectroscopic investigations and radio astronomical searches.
Preparation
Palladium catalyzed synthesis of phenanthridine has been accomplished by Pritchard and coworkers from imidoyl selenides. This was the first report of palladium insertion into the C-Se bond. The palladium insertion into the imidoyl selenides 3.20 followed by intramolecular cyclization and subsequent aromatization via the elimination of HSePh lead to the formation of substituted phenanthridines 3.21. 
Pd-catalysed synthesis of phenanathridine
Definition
ChEBI: Phenanthridine is an azaarene that is the 9-aza derivative of phenanthrene. The parent of the class of phenanthridines. It is a mancude organic heterotricyclic parent, a polycyclic heteroarene, a member of phenanthridines and an azaarene.
Synthesis Reference(s)
Journal of the American Chemical Society, 74, p. 6295, 1952 DOI: 10.1021/ja01144a521
Journal of Heterocyclic Chemistry, 26, p. 865, 1989 DOI: 10.1002/jhet.5570260366
Tetrahedron Letters, 29, p. 5463, 1988 DOI: 10.1016/S0040-4039(00)80787-X
General Description
Phenanthridine appears as crystalline needles. Mutagenic.
Air & Water Reactions
Sparingly soluble in water.
Reactivity Profile
PHENANTHRIDINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data are not available for PHENANTHRIDINE, but is probably combustible.
Safety Profile
Mutation data reported. When heated to decomposition it emits toxic vapors of NOx.
Properties of Phenanthridine
| Melting point: | 104-107 °C (lit.) |
| Boiling point: | 349 °C/769 mmHg (lit.) |
| Density | 1.1255 (rough estimate) |
| refractive index | 1.7270 (estimate) |
| Flash point: | 100 °C |
| solubility | phosphate buffer: soluble |
| form | Powder |
| pka | 5.58(at 20℃) |
| color | White to beige |
| Water Solubility | 0.3g/L(20 ºC) |
| BRN | 120204 |
| CAS DataBase Reference | 229-87-8(CAS DataBase Reference) |
| EPA Substance Registry System | Phenanthridine (229-87-8) |
Safety information for Phenanthridine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Phenanthridine
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran


You may like
-
 Phenanthridine CAS 229-87-8View Details
Phenanthridine CAS 229-87-8View Details
229-87-8 -
 Phenanthridine 95% CAS 229-87-8View Details
Phenanthridine 95% CAS 229-87-8View Details
229-87-8 -
 Phenanthridine CAS 229-87-8View Details
Phenanthridine CAS 229-87-8View Details
229-87-8 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
