pecilocin
- CAS NO.:19504-77-9
- Empirical Formula: C17H25NO3
- Molecular Weight: 291.388
- MDL number: MFCD00137492
- EINECS: 2431169
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-16 17:08:46
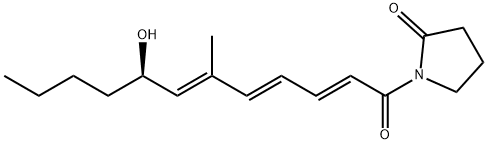
What is pecilocin?
Description
Pecilocin was discovered in the culture broth and mycelium of Paecilomyces varioti var. antibioticus by Yonehara et al. of the University of Tokyo in 1959. It has an oily nature and shows activity against specific filamentous fungi. Pecilocin is used as an ointment or in ethanolic solution for the treatment of dermatomycoses caused by Trichophyton, Microsporum, and Epidermophyton.
Originator
Variotin,Shanghai Lansheng Corporation
The Uses of pecilocin
Variotin is JAN.
Definition
ChEBI: Variotin is a N-acylpyrrolidine.
Manufacturing Process
Paecilomyces varioti Bainier var. antibioticus ATCC 13435 was inoculated into 10 liters of a culture medium having a pH of 6 and containing 3.0% sucrose, 0.3% sodium nitrate, 0.2% potassium dihydrogen phosphate, 0.05% magnesium sulfate, 0.05% potassium chloride and 0.001% ferrous sulfate. The cultivation was carried out in a small-aerated tank at a temperature of 25°C. After cultivation for 60 hours, the production of 30 units of pecilocin (variotin) was accomplished. The fermentation broth was then separated from the mycelium by filtration and the filtrate was extracted twice with 3 litres of ethyl acetate. The combined extracts were concentrated under reduced pressure. The concentrate was dissolved into 100 ml of methanol and, after filtering off the insoluble material which formed on refrigeration of the resulting solution, the methanolic solution was concentrated under reduced pressure. Thus 1.8 g of pecilocin having an activity of 120 u/mg were obtained.
The mycelium separated from the fermentation broth by filtration was treated with 1 liter of methanol and, after thorough grinding and stirring, was centrifugally separated. The methanol was distilled off from the methanol extract under reduced pressure and the residue extracted with ethyl acetate. The extract was concentrated under reduced pressure and the resulting concentrate was dissolved in about 100 ml of methanol. After removing the insoluble materials, which appeared on refrigeration, the methanol solution was concentrated under reduced pressure and 0.8 g of pecilocin having an activity of 90 u/mg were obtained.
100 liters of the same medium as used above were charged into the 200 liters fermentation tank. 50 g of steamed rice which had been inoculated with Paecilomyces varioti Bainier var. antibioticus ATCC 13435 and fully sporulated after cultivation for a week were seeded in the tank and cultivated with aeration and agitation at a temperature of 26°-27°C for 90 hours, said aeration being carried out by sparging of sterilized air at the rate of 90 liters per minute. At the end of 90 hours, the fermentation broth showed a pecilocin content of 16 u/ml. 86 liters of the cultured solution including the mycelium were extracted twice with 30 liters of ethyl acetate and centrifuged in a Sharples centrifugal machine. The combined extracts were concentrated under reduced pressure and about 55 g of brownish colored syrup were obtained. This syrup was dissolved in 250 ml of methanol and then refrigerated. The insoluble materials, which appeared were removed by filtration. The clarified methanol solution was then concentrated under reduced pressure and the resulting syrup was dissolved in ether and the insoluble materials were filtered off. The ether solution was concentrated under reduced pressure to a volume of about 25 ml and the concentrate mixed with ten times its volume of
petroleum ether and refrigerated. Oily material, which precipitated were separated from the solvent by decantation and washed with a small volume of petroleum ether. After drying, the treated oily materials were dissolved in 300 ml of carbon tetrachloride and then refrigerated. Brownish-red colored oily materials, which had formed were removed by decantation and the solution in carbon tetrachloride was concentrated under reduced pressure, thereby 6.6 g of a slightly yellow oily substance having an activity of 145 μ/mg were obtained.
One gram of this oily substance was subjected to a 47 tube counter-current distribution employing a 1:1 mixture of 70% methanol and carbon tetrachloride as the solvent. The results of bio-assay, ultra-violet absorption and weight measurements showed that the biologically active component was distributed mainly in tubes No 12 - 32 and that tube No 21 showed the highest concentration of active component. The samples of tubes No 15 - 26 were combined and again counter-currently distributed, 130 tubes being used. As a result of this counter-current distribution, the pecilocin was distributed in tubes No 47 - 73 of which tube No 61 showed the highest content of pecilocin. Distribution curves were plotted from bio-assay, UV-absorption and weight measurements and these curves agreed well with theoretical curve. It was thus proved that variation is a single substance. The samples of tubes No 58 - 63 were combined and concentrated under reduced pressure whereby 110 mg of colorless oily substance having a pecilocin activity of 166 u/mg were obtained.
Therapeutic Function
Antibiotic
Properties of pecilocin
| Melting point: | 41.5-42.5 °C |
| alpha | D28 -5.68° (methanol) |
| Boiling point: | 481.2±37.0 °C(Predicted) |
| Density | 1.088±0.06 g/cm3(Predicted) |
| pka | 14.11±0.20(Predicted) |
Safety information for pecilocin
Computed Descriptors for pecilocin
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1