oxyacanthine
- CAS NO.:548-40-3
- Empirical Formula: C37H40N2O6
- Molecular Weight: 608.72
- EINECS: 208-946-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
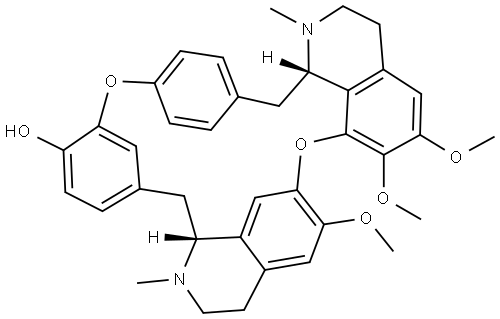
What is oxyacanthine?
Description
This alkaloid was first obtained by Hesse from Berberis vulgaris and is also a minor constituent of other Berberis and Mahonia species. When recrystallized from EtOH it forms clusters of colourless needles, m.p. 208-214°C, the higher melting point given above being obtained in vacuo. It has [α]D + 131.6° (CHC13), a much higher value of + 279° (CHCI3) being given by Gericke and Bruchhausen. The dihydrochloride forms needles, m.p. 270-1°C (vac.); [α] +29 D+ 188.5° (H20) and is only sparingly soluble in dilute HC1; the hydrobromide has m.p. 273-5°C (dec., vac.) and the nitrate forms colourless needles, m.p. 195- 200°C (dec.). The presence of the phenolic hydroxyl group is indicated by the formation of an O-benzoy1 derivative, a potassium salt and the O-methyl ether, giving a hydrochloride, m.p. 261°C. The structure has been deduced from chem_x0002_ical and spectroscopic data. Various colour reactions have been described, e.g. the alkaloid is not coloured by H2S04, gives a yellow colour with HN03 and with molybdic acid in H2S04 produces a violet colour, slowly changing to yellow-green.
Definition
ChEBI: A macrocycle that is oxyacanthan that is substituted by methoxy groups at positions 6, 6', and 7, methyl groups at positions 2 and 2', and a hydroxy group at the 12' position.
Properties of oxyacanthine
| Melting point: | 216-217° |
| Boiling point: | 655.15°C (rough estimate) |
| alpha | D20 +131.5° (chloroform) |
| Density | 1.1648 (rough estimate) |
| refractive index | 1.5300 (estimate) |
| pka | 9.42±0.20(Predicted) |
Safety information for oxyacanthine
Computed Descriptors for oxyacanthine
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateYou may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
