Oxetorone
- CAS NO.:34522-46-8
- Empirical Formula: C21H21NO2.C4H4O4
- Molecular Weight: 435.474
- MDL number: MFCD01698570
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-04 19:55:51
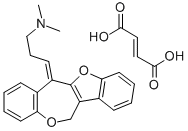
What is Oxetorone?
Originator
Nocertone, Labaz , France ,1975
The Uses of Oxetorone
Analgesic (specific in migraine).
Manufacturing Process
(A) Preparation of 6-(3-dimethylaminopropyl)-6-hydroxybenzo[b]benzofurano [2,3-e]oxepin - In a 250 ml flask equipped with a vertical condenser, a dropping-funnel, a dip thermometer and a stirrer, 1.5 g of magnesium turnings and a crystal of iodine were heated until vaporization of the iodine and then cooled, after which 20 ml of dry tetrahydrofuran were added.
The mixture was heated under reflux and a solution of 0.2 g of ethyl iodide in 5 ml of dry tetrahydrofuran was allowed to flow into the reaction medium. When the reaction started, a solution of 6.2 g of γ-dimethylaminopropyl chloride in 20 ml of dry tetrahydrofuran was added and the mixture so obtained was heated under reflux until the complete disappearance of the magnesium turnings. The reaction medium was then cooled in an ice bath, after which there was added thereto a solution in 45 ml of tetrahydrofuran of 7 g of 6-oxo-benzo[bl]-benzofurano[2,3-e]oxepin. The reaction mixture was allowed to stand for 20 hours at a temperature of 20°C, and was then poured into a saturated aqueous solution of ammonium chloride maintained at a temperature of 5°C. The mixture was extracted with ether and the organic portion was washed and dried over anhydrous sodium sulfate. After evaporation of the solvent, 9.4 g of crude product were obtained, which after recrystallization from isopropanol, provided 6.7 g of pure 6-(3dimethylaminopropyl)-6-hydroxybenzo[b]benzofurano[2,3-e]oxepin, melting
point 160°C (yield, 71%).
(8) Preparation of 6-(3-dimethylaminopropylidene)-benzo[b]benzofurano[2,3e]oxepin and its fumarate -In an Erlenmeyer flask 6.2 g of 6-(3dimethylaminopropyl)-6-hydroxybenzo[b]benzofurano[2,3-e]oxepin prepared as described above were dissolved in 108 ml of a 10% solution of sulfuric acid. The solution obtained was heated to boiling point for 15 minutes. After cooling, 100 ml of chloroform were added and the solution was made alkaline with a 5% solution of sodium hydroxide. The solution was then extracted with chloroform, washed with water and dried over anhydrous sodium sulfate. The solvent was evaporated and the resulting oily residue composed of 6-(3dimethylaminopropylidene)-benzo[b]benzofurano[2,3-e]oxepin was then directly treated with a solution of fumaric acid in isopropanol to give 6.5 g of 6-(3-dimethylaminopropylidene)-benzo[b]benzofurano[2,3-e]oxepin fumarate (yield, 85%). The fumarate had a melting point of 160°C when recrystallized from isopropanol.
brand name
. Nocertone (Labaz S.A., France).
Therapeutic Function
Serotonin antagonist, Antihistaminic
Properties of Oxetorone
| Melting point: | 160° |
Safety information for Oxetorone
Computed Descriptors for Oxetorone
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
