Olmutinib
- CAS NO.:1353550-13-6
- Empirical Formula: C26H26N6O2S
- Molecular Weight: 486.59
- MDL number: MFCD29918158
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:18:20
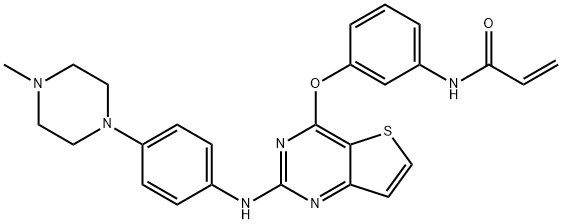
What is Olmutinib?
Description
Olmutinib, codeveloped by Boehringer Ingelheim and Hanmi Pharmaceutical Co., was approved by the Korean Ministry of Food and Drug Safety (MFDS) for treatment of locally advanced or metastatic EGFR T790 M mutation-positive non-small cell lung cancer (NSCLC). Olmutinib serves as a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI), which is being used as an oral therapy for patients who have previously been treated with an alternate EGFR TKI. Firstand second-generation EGFR TKIs, which bind reversibly and irreversibly to the TK domain, respectively, are both generally effective at onset of treatment but result in development of resistance within the first year of treatment. Thirdgeneration EGFR TKIs such as olmutinib have demonstrated the ability to covalently bind to the kinase domain of EGFR while sparing wild-type EGFR, leading to irreversible inhibition of both EGFR mutations and the T790 M mutation, which is linked to EGFR TKI resistance.
Description
HM61713 is a third generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor that inhibits both EGFR activating and T790M resistance mutations, while sparing wild-type EGFR. It has been investigated in Phase II clinical trials in patients with EGFR T790M mutation-positive non-small cell lung cancer (NSCLC), who developed resistance to earlier generations of EGFR tyrosine kinase inhibitor therapy.
The Uses of Olmutinib
Olmutinib is an epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) against non-small cell lung cancer (NSCLC).
Synthesis
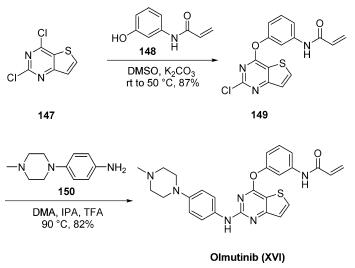
Synthetically, olmutinib can be accessed in two steps beginning with 2,4-dichloro-thieno[3,2-d]pyrimidine (147), which is commercially available and can be prepared in two steps from urea and 3-aminothiophene 2- carboxylate. Nucleophilic addition of N-(3-hydroxyphenyl)-2- propenamide (148) to 147 proceeded with complete regioselectivity via treatment with K2CO3 in warm DMSO, smoothly providing the desired diaryl ether 149 in 87% yield after crystallization from isopropanol and water. From 149, substitution with commercially available piperazinyl aniline 150 under acidic heating conditions (DMA, IPA, TFA, 90 ??C) provided olmutinib in 82% yield. After recrystallization, olmutinib (XVI) was obtained in 60% overall yield and 99.1% purity.
in vitro
olmutinib has been identified as an irreversible kinase inhibitor and could covalently bind to a cysteine residue near the kinase domain of mutant egfr. olmutinib had a half-life of over 24h for egfr inhibition. olmutinib was able to cause potent inhibition in cell lines h1975 (l858r and t790m) and hcc827 (exon 19 deletion). olmutinib showed a low potency for nsclc cell line h358 with wild-type egfr [1].
in vivo
the previous in vivo studies of xenograft models with grafts of h1975 and hcc827 showed that olmutinib was active against the tumors without dasplaying any side effects [1].
References
[1] wang s, cang s, liu d. third-generation inhibitors targeting egfr t790m mutation in advanced non-small cell lung cancer. j hematol oncol. 2016 apr 12;9:34.
Properties of Olmutinib
| Density | 1.336±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; ≥9.11 mg/mL in EtOH with ultrasonic; ≥96.6 mg/mL in DMSO |
| form | crystalline solid |
| pka | 12.87±0.70(Predicted) |
| color | Light yellow to yellow |
Safety information for Olmutinib
Computed Descriptors for Olmutinib
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Olmutinib 95% CAS 1353550-13-6View Details
Olmutinib 95% CAS 1353550-13-6View Details
1353550-13-6 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5