OBIDOXIME CHLORIDE
Synonym(s):1,1′-(Oxydimethylene)bis(pyridinium-4-carbaldoxime) dichloride;Bis(4-formylpyridiniomethyl) ether dioxime;Toxogonin
- CAS NO.:114-90-9
- Empirical Formula: C14H16Cl2N4O3
- Molecular Weight: 359.21
- MDL number: MFCD00038864
- EINECS: 204-059-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-16 16:10:54
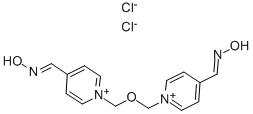
What is OBIDOXIME CHLORIDE?
Chemical properties
White Crystalline Solid
Originator
Toksobidin,Polfa-Starogard
The Uses of OBIDOXIME CHLORIDE
Obidoxime Chloride is a cholinesterase reactivator.
The Uses of OBIDOXIME CHLORIDE
antibacterial
What are the applications of Application
Obidoxime Chloride is a cholinesterase reactivator
Manufacturing Process
2 Methods of producing of obidoxime chloride:
1. Into a boiling agitated solution of 2.44 g pyridine-4-aldoxime in 10 ml absolute ethanol is added dropwise during the course of 25 min a solution of 1.14 g bis-chloromethyl ether in 5 ml absolute ethanol. The reaction mixture is then refluxed for 35 min, and then agitated for 5 h at room temperature. The precipitate of bis-[4-hydroxyimino-methyl-pyridinium-(1)-methyl]-ether
Trade dichloride is thoroughly washed with absolute acetone. The yield is 3.5 g which is 98% of the theoretical, and the melting point is 229°C. If convenient, the mother liquor can be reused to make additional product.
2. 12.2 g (0.1 mole) pyridine-4-aldoxime are dissolved with heating in 125 ml chloroform. Within 25 min, 8.5 g (0.075 mole), α,α-dichlorodimethyl ether in 20 ml chloroform are dropped while stirring into the boiling solution. The reaction mixture is heated for another 35 min. After standing for several hours, the precipitate is filtered off, washed with absolute ethanol, acetone and ether and dried at 80°C. Yield: 17.0 g, 95% of the theoretical, and the melting point is 225°C (dec.).
Therapeutic Function
Antidote
General Description
active agent in Toxogonin
Biochem/physiol Actions
Antidote for organophosphate nerve agent poisoning, but, as with other oxime agents, not full spectrum. Obidoxime fails primarily to reactivate acetylcholinesterase that has been inhibited with cyclosarin.
Safety Profile
Poison by intraperitoneal,intravenous, intramuscular, and subcutaneous routes.Moderately toxic by ingestion. When heated todecomposition it emits toxic fumes of Cl- and NOx. Seealso BROMIDES.
Properties of OBIDOXIME CHLORIDE
| Melting point: | 216-219oC dec. |
| storage temp. | -20°C Freezer |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | neat |
| color | Off-White to Light Beige |
| BRN | 4117377 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
Safety information for OBIDOXIME CHLORIDE
Computed Descriptors for OBIDOXIME CHLORIDE
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 114-90-9 Obidoxime chloride 98%View Details
114-90-9 Obidoxime chloride 98%View Details
114-90-9 -
 114-90-9 Obidoxime chloride 99%View Details
114-90-9 Obidoxime chloride 99%View Details
114-90-9 -
 Obidoxime chloride CAS 114-90-9View Details
Obidoxime chloride CAS 114-90-9View Details
114-90-9 -
 Obidoxime chloride CAS 114-90-9View Details
Obidoxime chloride CAS 114-90-9View Details
114-90-9 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
