nifurzide
- CAS NO.:39978-42-2
- Empirical Formula: C12H8N4O6S
- Molecular Weight: 336.28
- MDL number: MFCD00865988
- EINECS: 2547280
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:38
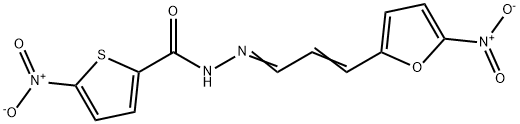
What is nifurzide?
Originator
Ricridene,Anphar,Switz.,1981
Definition
ChEBI: Nifurzide is a member of thiophenes and a C-nitro compound.
Manufacturing Process
(a) Ethyl-5-nitro-2-thiophene carboxylate:
17.4 g (mol/10 = 17.31 g) of 5-nitrothiophene carboxylic acid are dissolved in
85 ml of absolute ethanol. A stream of gaseous hydrochloric acid is caused to
enter the boiling solution to the point of saturation, and for 5 hours.
Evaporation to dryness takes place and then the solid residue is washed with
a sodium bicarbonate solution. It is suction-filtered and washed with water.
After drying, there are obtained 17.7 g of a yellow product with a melting
point of 63°C to 65°C and the yield is 88% (theoretical yield = 88%).
The N'-(5'-nitro-2'-thenoyl)hydrazide is prepared by reacting hydrazine with
ethyl 5-nitro-2-thiophene carboxylate.
(b) 6.3 g (mol/30 = 6.5 g) of N1-[5'-nitro-2'-thenoyl]hydrazide are dissolved
in 100 ml of dry tetrahydrofuran. 5.6 g (mol/30 = 5.55 g) of 5-nitro-2-furyl
acrolein in 56 ml of tetrahydrofuran are added. Heating under reflux takes
place for 1 hour and, 25 minutes after starting the heating, the crystallization
commences; the crystals are suction-filtered, washed with ether and dried.
There are obtained 7.9 g (yield 70%-theoretical yield = 11.2 g) of a yellow
solid of melting point 235°C to 236°C.
Recrystallization (tepid dimethylformamide + ether) leaves the melting point
unchanged.
Therapeutic Function
Antibacterial, Antidiarrheal
Properties of nifurzide
| Melting point: | 235-236° |
| Density | 1.62±0.1 g/cm3(Predicted) |
| pka | 10.31±0.46(Predicted) |
Safety information for nifurzide
Computed Descriptors for nifurzide
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran

You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 4-Bromo Benzylcyanide 99%View Details
4-Bromo Benzylcyanide 99%View Details
26451-08-1 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
