narciclasine
Synonym(s):Narciclasine;Lycoricidinol;(2S-(2-alpha,3-beta,4-beta,4a-beta))-3,4,4a,5-tetrahydro-2,3,4,7-tetrahydroxy-(1,3)Dioxolo(4,5-j)phenanthridin-6(2H)-one;3,4,4a,5-Tetrahydro-2,3,4,7-tetrahydroxy-(1,3)dioxolo(4,5-j)phenanthridin-6(2H)-one;Lycoricidin-A
- CAS NO.:29477-83-6
- Empirical Formula: C14H13NO7
- Molecular Weight: 307.26
- MDL number: MFCD01729949
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-07 22:15:30
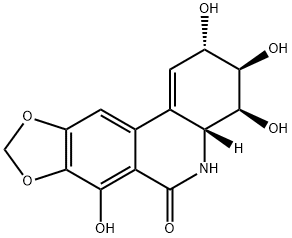
What is narciclasine?
Description
Narciclasine is a plant growth inhibitor that can be isolated from Narcissus bulbs. At 1 μM, it has been shown to induce apoptosis-mediated cytotoxicity in human cancer cells in vitro but not in normal fibroblasts. Narciclasine has been shown to regulate the Rho/Rho kinase/LIM kinase/cofilin signaling pathway by increasing GTPase RhoA activity, as well as inducing actin stress fiber formation in a RhoA-dependent manner.
Description
Narciclasine, also known as lycoricidinol, is an isocarbostyril alkaloid found in the Amaryllidaceae (amaryllis) family of flowering plants. One notable genus of the family is Narcissus, from which narciclasine gets its name. Daffodils and jonquils are members of the Narcissus genus.
In 1967, Giovanni Ceriotti, Luigi Spandrio, and Annivale Gazzaniga at Circolo Hospital (Busto Arsizio, Italy) isolated narciclasine from Narcissus varieties. At that time, it was known to inhibit cell division. By the early 2010s, narciclasine was an established antitumor agent.
In 2016, Robert Fürst at Goethe University (Frankfurt am Main, Germany) reviewed the antitumor and anti-inflammatory findings on narciclasine. At present, no clinical trials on narciclasine have been conducted on humans.
Narciclasine is sparingly soluble in water. But in 2003, George R. Petit and colleagues at Arizona State University (Tempe) synthesized a cyclic phosphate at two of the adjacent hydroxyl groups of narciclasine. The product, which they called narcistatin (CAS Reg. no. 496963-44-1), is much more water-soluble (4 g/L), making it a valuable prodrug for narciclasine.
Description
The bulbs of several Narcissus species contain this alkaloid which crystallizes in the form of pale yellow needles with a pronounced yellow-green fluorescence. It is best purified by recrystallization from either acetic acid or an aqueous mixture of methoxyethyl alcohol. It is dextrorotatory with [α]589 + 145° or [Qh64 + 983° (c 1.5, EtOH). The ultraviolet spectrum in neutral solution (EtOH) has absorption maxima at 252, 302 and 329 mil, while that in alkaline solution (0.01 N/NaOH) has the maxima at 219, 249,310 and 355 mil. The O-methyl ether forms shiny needles which also exhibit a strong blue fluorescence. The tri_x0002_acetate has been prepared as an amorphous, non-crystallizable substance. With FeC13 the alkaloid gives a violet colour.
The Uses of narciclasine
Narciclasine is reasonably abundant in some Narcissus spp. and has served as a very useful intermediate for synthetic conversion into (+)-pancratistatin and to conduct a series of structure–activity relationship studies . Bicolorine, another member of the narciclasine series, is an unusual, completely aromatized quaternary alkaloid with an N-methyl group.
The Uses of narciclasine
Narciclasine is an antiproliferative and pro-apoptotic inducer.
What are the applications of Application
Narciclasine is an antiproliferative and pro-apoptotic inducer
Definition
ChEBI: A natural product found in Narcissus pseudonarcissus.
Storage
Store at -20°C
References
Ceriotti., Nature, 213,595 (1967)
Piozzi et al., Tetrahedron, 24, 1119 (1968)
Revised structure:
Mondon, Krohn., Tetrahedron Lett., 2123 (1970)
Savona, Piozzi, Marino., Chern. Cornrnun., 1006 (1970)
Absolute configuration:
Fuganti, Mazza.,J. Chern. Soc., Chern. Cornrnun., 239 (1972)
Crystal structure:
Immirzi, Fuganti., J. Chern. Soc., Chern. Cornrnun., 240 (1972)
Stereochemistry:
Mondon, Krohn., Tetrahedron Lett., 2085 (1972)
Properties of narciclasine
| Melting point: | 250-252℃ |
| Boiling point: | 447.77°C (rough estimate) |
| Density | 1.85±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5300 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMSO: ≥10mg/mL |
| pka | 7.72±0.70(Predicted) |
| form | Solid |
| appearance | white to off-white powder |
| color | white to off-white |
Safety information for narciclasine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for narciclasine
New Products
DL-beta-(3-Bromophenyl)alanine 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Amlodipine EP Impurity-C Budesonide Impurity-K/Budesonide 21-Acetate (Epimers) Gabapentine Impurity 72 Bicalutamide Impurity-B Betahistine EP Impurity C Carbamazepine EP Impurity G 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3-Hydroxypropionitrile 4-Bromo Benzylcyanide valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Narciclasine CAS 29477-83-6View Details
Narciclasine CAS 29477-83-6View Details
29477-83-6 -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 4-Bromo Benzylcyanide 99%View Details
4-Bromo Benzylcyanide 99%View Details
26451-08-1 -
 3-Hydroxypropionitrile 109-78-4 99%View Details
3-Hydroxypropionitrile 109-78-4 99%View Details
109-78-4 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
