Nafenopin
- CAS NO.:3771-19-5
- Empirical Formula: C20H22O3
- Molecular Weight: 310.39
- MDL number: MFCD00865724
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 11:31:08
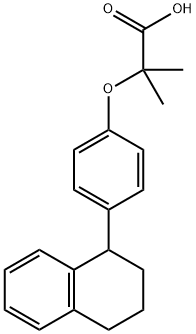
What is Nafenopin?
Definition
ChEBI: A monocarboxylic acid that is 2-hydroxy-2-methylpropanoic acid in which ther tertiary hydroxy group has been converted to the corresponding p-(1,2,3,4-tetrahydronaphthalen-1-yl)phenyl ether.
General Description
Crystals.
Reactivity Profile
An organic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Nafenopin to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible.
Fire Hazard
Flash point data for Nafenopin are not available. Nafenopin is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Moderately toxic by ingestion. Mutation data reported. A drug for the treatment of hypercholesterolemia or hypertriglyceridemia. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of Nafenopin
| Melting point: | 117-118 °C |
| Boiling point: | 390.55°C (rough estimate) |
| Density | 1.1662 (rough estimate) |
| refractive index | 1.4618 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| pka | 3.28±0.10(Predicted) |
| IARC | 2B (Vol. 24, Sup 7) 1987 |
| EPA Substance Registry System | Nafenopin (3771-19-5) |
Safety information for Nafenopin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H351:Carcinogenicity H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nafenopin
New Products
4-Iodo-3,5-dimethylbenzonitrile 2-fluoro-4-iodoaniline 2-(3-Methyl-1,2,4-oxadiazol-5-yl)acetic acid (as potassium salt) 6,6'-methylenebis(9-methyl-3-((2-methyl-1H-imidazol-1-yl)methyl)-2,3-dihydro-1H-carbazol-4(9H)-one) 3-Bromo-2-fluorobenzoic acid Quinuclidine-4-carbonitrile N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (R)-1-Benzyl-3-pyrrolidinecarbonitrile Bupropian related compound F Lantanoprost rc B Clidinium Bromide Impurity Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity N-Nitroso hydroxy Cetrizine EP Impurity-A 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 1,4-bis(methylsulfonyl)butane 4-Cyano-N-Methacryloyl-3-Trifluoromethyl Aniline 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzeneRelated products of tetrahydrofuran


You may like
-
![6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
6,6'-bis(difluoromethoxy)-2,2'-bis((3,4-dimethoxypyridin-2-yl)methylsulfinyl)-1H,1'H-5,5'-bibenzo[d]imidazole 98%View Details
2115779-15-0 -
 4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
4-bromo-1H-pyrazole-3-carboxylic acid 98%View Details
13745-17-0 -
 230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 (1-((2'-(2-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-72-9 -
 1941177-45-2 98%View Details
1941177-45-2 98%View Details
1941177-45-2 -
 Isopropyl amine Hydrochloride 99%View Details
Isopropyl amine Hydrochloride 99%View Details
15572-56-2 -
 19501-58-7 99%View Details
19501-58-7 99%View Details
19501-58-7 -
 Cyclopropane-1,1-dicarboxylic acid 99%View Details
Cyclopropane-1,1-dicarboxylic acid 99%View Details
598-10-7 -
 230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8 (1-((2'-(1-((1-((2'-(2H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methyl)-1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-butyl-4-chloro-1H-imidazol-5-yl)methanol 98%View Details
230971-71-8
