misonidazole
- CAS NO.:13551-87-6
- Empirical Formula: C7H11N3O4
- Molecular Weight: 201.181
- MDL number: MFCD00866600
- EINECS: 2369316
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-27 08:46:37
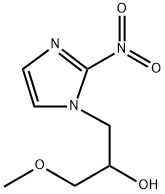
What is misonidazole?
Description
Misonidazole is a nitroimidazole with radiosensitizing and antineoplastic properties. Nitroimidazoles, including misonidazole, specifically accumulate as nitro anion radicals and their metabolites in hypoxic cells. The metabolites of misonidazole can themselves be cytotoxic or they can increase the chemosensitivity and radiosensitivity of the target cells. Misonidazole and derivatives, including fluoromisonidazole, are used in the imaging of hypoxic regions in tumors and in the cardiovascular system.
The Uses of misonidazole
Misonidazole can be used as a potential hypoxic cancer cell radiosensitizer and DNA binding and nicking agent. The metabolites of misonidazole can themselves be cytotoxic or they can increase the chemosensitivity and radiosensitivity of the target cells
The Uses of misonidazole
Antiprotozoal (Trichomonas).
in vitro
in cultured cho cells, pretreatment with 5 mm miso for 2 hr exihibited the marginal toxicity [2].
in vivo
pretreatment with misonidazole (miso) enhanced the number of cross-links formed in a fibrosarcoma and in the spleen and gut of mice for periods up to 48 h following a single injection of melphalan (mel). miso pretreatment could result in a greater amount of binding of mel to dna at early times after injection. miso may exert its affect by inhibiting the repair of cross-links or monoadducts at early times post-injection [3].
References
[1] josephy p d, palcic b, skarsgard l d. ascorbate-enhanced cytotoxicity of misonidazole[j]. nature, 1978, 271(5643): 370-372.
[2]taylor y c, bump e a, brown j m. studies on the mechanism of chemosensitization by misonidazole in vitro[j]. international journal of radiation oncology biology physics, 1982, 8(3-4): 705-708.
[3] murray d, meyn r e. enhancement of the dna cross-linking activity of melphalan by misonidazole in vivo[j]. british journal of cancer, 1983, 47(2): 195.
[4] wasserman t h, stetz j a, phillips t l.
Properties of misonidazole
| Melting point: | 107-109°C |
| Boiling point: | 339.09°C (rough estimate) |
| Density | 1.3983 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly, Heated), Methanol (Slightly, Sonicated) |
| pka | 13.49±0.20(Predicted) |
| form | Solid |
| color | Yellow to Orange |
Safety information for misonidazole
Computed Descriptors for misonidazole
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran

![2-NITRO-ALPHA-[(2,2,2-TRIFLUOROETHOXY)METHYL]-IMIDAZOLE-1-ETHANOL](https://img.chemicalbook.in/CAS/GIF/21787-91-7.gif)
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 4-Bromo Benzylcyanide 99%View Details
4-Bromo Benzylcyanide 99%View Details
26451-08-1 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
