Maleic anhydride
Synonym(s):2,5-Furandione;2,5-Furanedione;Maleic anhydride
- CAS NO.:108-31-6
- Empirical Formula: C4H2O3
- Molecular Weight: 98.06
- MDL number: MFCD00005518
- EINECS: 203-571-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-30 17:41:12
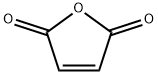
What is Maleic anhydride?
Description
Maleic anhydride (MAN), with the chemical formula C?H?O?, is the acid anhydride of maleic acid and appears as a colorless or white solid with an acrid odor. Its multifunctional molecular structure makes it uniquely valuable in chemical synthesis and industrial applications. As a key chemical intermediate, it is primarily used in the production of unsaturated polyester resins (UPR), which are widely employed in reinforced and unreinforced materials for construction, marine, and automotive industries. Additionally, it serves as a critical raw material for synthesizing 1,4-butanediol (BDO), gamma-butyrolactone, and tetrahydrofuran (THF), with BDO being one of the world's fastest-growing chemicals in recent years.
Description
Maleic anhydride is perhaps best known as the quintessential dienophile in the Diels–Alder cycloaddition reaction, but it is also used as a ligand in metal complexes and as a feedstock for maleic acid and its half-esters. It was first synthesized from the acid by R. Kempf in 1908. Until recently, commercial production used benzene as the feedstock; but for health and safety reasons, newer plants use butane.
Chemical properties
Maleic anhydride is colorless needles, white lumps, or pellets. Irritating, choking odor. Dissolves in water to produce maleic acid. Dissolves in ethanol and produces esters.
Physical properties
White, hydroscopic crystals (usually shipped as briquettes). Odor threshold concentration is 0.32 ppm (quoted, Amoore and Hautala, 1983).
The Uses of Maleic anhydride
In the manufacture of polyester resins, fumaric acid, agricultural pesticides, and alkyl resins
Definition
ChEBI: Maleic anhydride is a cyclic dicarboxylic anhydride that is the cyclic anhydride of maleic acid. It has a role as an allergen. It is a cyclic dicarboxylic anhydride and a member of furans.
Production Methods
Maleic anhydride was traditionally manufactured by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller plants continue to use benzene; due to rising benzene prices, most maleic anhydride plants now use n-butane as a feedstock.
In both cases, benzene and butane are fed into a stream of hot air, and the mixture is passed through a catalyst bed at high temperature. The ratio of air to hydrocarbon is controlled to prevent the mixture from catching on fire. Vanadium pentoxide and molybdenum trioxide are the catalysts used for the benzene route, whereas vanadium and phosphorus oxides are used for the butane route.
2 CH3CH2CH2CH3 + 7 O2 → 2 C2H2(CO)2O + 8 H2O.
Preparation
A flask equipped with a Dean-Stark trap, condenser, and mechanical stirrer was charged with 116 g (1.0 mol) of maleic acid and 120 mL of tetrachloroethane. The mixture was heated to remove water (18 mL, 1.0 mol) via azeotropic distillation, and the residue was distilled under reduced pressure to yield 87.7 g (89.5%) of maleic anhydride, b.p. 82–84°C (15 mmHg), m.p. 53°C. The flask residue contained approximately 10 g of fumaric acid (m.p. 287°C). Both fumaric and maleic acids form maleic anhydride upon heating, though fumaric acid requires higher temperatures to first isomerize to maleic acid before dehydration.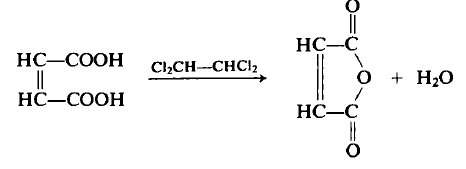
Reactions
The chemistry of maleic anhydride is very rich, reflecting its ready availability and bifunctional reactivity. It hydrolyzes, producing maleic acid, cis-HOOC–CH=CH–COOH. With alcohols, the halfester is generated, e.g., cis-HOOC–CH=CH–COOCH3.
Maleic anhydride is a potent dienophile in Diels-Alder reactions. It is also a ligand for low-valent metal complexes, examples being Pt(PPh3)2(MA) and Fe(CO)4(MA).
Maleic anhydride dimerizes in a photochemical reaction to form cyclo butane tetra carboxylic dianhydride (CBTA). This compound is used in the production of polyimides and as an alignment film for liquid crystal displays.
General Description
Maleic anhydride is a colorless crystalline solid that may appear as needles, flakes, pellets, or a fused mass, with a melting point of 113°F. It is shipped in both solid and molten forms. Its vapors, fumes, and dust are highly irritating to the eyes, skin, and mucous membranes. It has a flash point of 218°F and an autoignition temperature of 890°F, and is primarily used in the manufacture of paints, plastics, and other chemicals.
Air & Water Reactions
Soluble in water. Reacts slowly with water to form maleic acid and heat.
Reactivity Profile
Maleic anhydride react vigorously on contact with oxidizing materials. Reacts exothermically with water or steam. Undergoes violent exothermic decomposition reactions, producing carbon dioxide, in the presence of strong bases (sodium hydroxide, potassium hydroxide, calcium hydroxide), alkali metals (lithium, sodium, potassium), aliphatic amines (dimethylamine, trimethylamine), aromatic amines (pyridine, quinoline) at temperatures above 150° C [Vogler, C. A. et al., J. Chem. Eng. Data, 1963, 8, p. 620]. A 0.1% solution of pyridine (or other tertiary amine) in Maleic anhydride at 185°C gives an exothermic decomposition with rapid evolution of gas [Chem Eng. News 42(8); 41 1964]. Maleic anhydride is known as an excellent dienophile in the Diels-Alder reaction to produce phthalate ester derivatives. These reactions can be extremely violent, as in the case of 1-methylsilacyclopentadiene [J. Organomet., Chem., 1979, 179, c19]. Maleic anhydride undergoes a potentially explosive exothermic Diels-Alder reaction with 1-methylsilacyclopenta-2,4-diene at 150C [Barton, T. J., J. Organomet. Chem., 1979, 179, C19], and is considered an excellent dieneophile for Diels-alder reactions [Felthouse, Timothy R. et al. "Maleic anhydride , Maleic Acid, and Fumaric Acid." Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2005].
Properties of Maleic anhydride
| Melting point: | 51-56 °C (lit.) |
| Boiling point: | 200 °C (lit.) |
| Density | 1.48 |
| vapor density | 3.4 (vs air) |
| vapor pressure | 0.16 mm Hg ( 20 °C) |
| refractive index | 1.4688 (estimate) |
| Flash point: | 218 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| pka | 0[at 20 ℃] |
| form | powder |
| color | White |
| PH | 0.8 (550g/l, H2O, 20℃)Hydrolysis |
| Odor | Mild acrid odor. |
| explosive limit | 1.4-7.1%(V) |
| Water Solubility | 79 g/100 mL (25 ºC) |
| Sensitive | Moisture Sensitive |
| Merck | 14,5704 |
| BRN | 106909 |
| Henry's Law Constant | (atm?m3/mol):
Not applicable - reacts with water |
| Exposure limits | NIOSH REL: TWA 1 ppm (0.25 mg/m3), IDLH 10 ppm; OSHA PEL: TWA
0.25 ppm; ACGIH TLV: TWA 0.25 ppm with an intended change of 0.1 ppm. |
| Dielectric constant | 51.0(60℃) |
| Stability: | Stable. Combustible. Incompatible with water, strong oxidizing agents, alkali metals, strong bases, amines, most common metals, polymerization catalysts and accelerators. |
| CAS DataBase Reference | 108-31-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,5-Furandione(108-31-6) |
| EPA Substance Registry System | Maleic anhydride (108-31-6) |
Safety information for Maleic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Maleic anhydride
| InChIKey | FPYJFEHAWHCUMM-UHFFFAOYSA-N |
Maleic anhydride manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Maleic Anhydride 99%View Details
Maleic Anhydride 99%View Details -
 Maleic Anhydride 99%View Details
Maleic Anhydride 99%View Details -
 MALEIC ANHYDRIDE 99%View Details
MALEIC ANHYDRIDE 99%View Details -
 Maleic Anhydride CAS 108-31-6View Details
Maleic Anhydride CAS 108-31-6View Details
108-31-6 -
 Maleic Anhydride CASView Details
Maleic Anhydride CASView Details -
 Maleic Anhydride CASView Details
Maleic Anhydride CASView Details -
 Maleic Anhydride Cas 108316View Details
Maleic Anhydride Cas 108316View Details
108-31-6 -
 Maleic Anhydride PowderView Details
Maleic Anhydride PowderView Details
108-31-6
