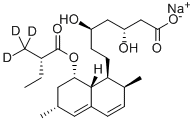
LOVASTATIN HYDROXY ACID, SODIUM SALT
- CAS NO.:75225-51-3
- Empirical Formula: C24H38O6
- Molecular Weight: 422.55
- MDL number: MFCD03425588
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
What is LOVASTATIN HYDROXY ACID, SODIUM SALT?
The Uses of LOVASTATIN HYDROXY ACID, SODIUM SALT
Lovastatin Hydroxy Acid, Sodium Salt (Lovastatin EP Impurity B) is a metabolite of Lovastatin.
Definition
ChEBI: A polyketide obtained by hydrolysis of the pyranone ring of lovastatin.
Properties of LOVASTATIN HYDROXY ACID, SODIUM SALT
| Melting point: | 140 - 143oC |
| Boiling point: | 602.3±55.0 °C(Predicted) |
| Density | 1.14±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly, Sonicated), Water (Slightly) |
| form | Solid |
| pka | 4.31±0.10(Predicted) |
| color | Off-White to Light Brown |
| Stability: | Very Hygroscopic |
Safety information for LOVASTATIN HYDROXY ACID, SODIUM SALT
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for LOVASTATIN HYDROXY ACID, SODIUM SALT
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran


![SIMVASTATIN ACID, SODIUM SALT [3H(G)]](https://img.chemicalbook.in/)



![LOVASTATIN ACID [3H(G)] SODIUM SALT](https://img.chemicalbook.in/StructureFile/ChemBookStructure8/GIF/CB6193357.gif)
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
