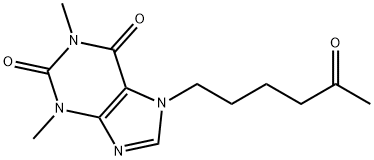
LOMIFYLLINE
- CAS NO.:10226-54-7
- Empirical Formula: C13H18N4O3
- Molecular Weight: 278.31
- MDL number: MFCD00867151
- EINECS: 233-547-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-05 11:31:08
What is LOMIFYLLINE?
Originator
Lomifylline,Yick-Vic Chemicals and
The Uses of LOMIFYLLINE
Lomifylline is a methylxanthine analogue which induces Ca2+-release from intracellular stores via the ryanodine receptor.
Definition
ChEBI: 1,3-dimethyl-7-(5-oxohexyl)purine-2,6-dione is an oxopurine.
Manufacturing Process
A mixture of 560 g of potassium carbonate, 700 ml of ethanol (96%), 404 g
of 1,3-dibromopropane and 260 g of ethyl acetoacetate was heated with
stirring to go 60°C. After the reaction had subsided, the reaction mixture was
refluxed for 5 hours. Then the bulk of the alcohol was distilled off under
ordinary pressure and the residue was mixed with 1.5 L of water. The
resulting oily layer was separated, and the aqueous phase was extracted with
benzene and the benzene layer was combined with the oil. After drying with
sodium sulfate the benzene was distilled off and the residue was fractionally
distilled 250 g (73% of theory) of 2-methyl-3-carbethoxy-5,6-dihydropyrane
of boiling point 105°-108°C were obtained.
140 ml of 63% hydrobromic acid were slowly added at room temperature to
128 g of 2-methyl-3-carbethoxy-5,6-dihydropyrane, and much carbon dioxidewas evolved. After standing for 1 to 2 days at room temperature the mixture
was diluted with an equal volume of iced water; the layer of dark colored oil
formed was separated, the aqueous phase was extracted with chloroform, and
the extract was combined with the oil and washed with a saturated solution of
sodium bicarbonate. The solution was dried with sodium sulfate, the
chloroform was distilled off under normal pressure, and the residue was
fractionally distilled in vacuo. 109 g (81% of theory) of 1-bromohexanone-5 of
boiling point 94°-98°C/12 mm Hg were obtained.
A solution of 10.0 g of 1-bromohexanone-5 in 100 ml of ethanol was gradually
mixed at the boil with vigorous stirring with 11.3 g of the sodium salt of
theophylline in 100 ml of water. After 3 hours refluxing the alcohol was
distilled off, and the residual aqueous phase was cooled and made alkaline
and extracted with chloroform. The chloroform solution was evaporated and
the residue re-crystallized from a little isopropanol to yield 7-(5-oxohexyl)
theophylline. MP: 75°-76°C; a yield of about 80% (calculated on the reacted
theophylline).
Therapeutic Function
Vasodilator
Metabolism
Not Available
Properties of LOMIFYLLINE
| Melting point: | 75-77°C |
| Boiling point: | 525.3±56.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 0.56±0.70(Predicted) |
| color | White to Off-White |
Safety information for LOMIFYLLINE
Computed Descriptors for LOMIFYLLINE
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 10226-54-7 1,3-Dimethyl-7-(5-oxohexyl)-1H-purine-2,6(3H,7H)-dione(Lomifylline API). 98%View Details
10226-54-7 1,3-Dimethyl-7-(5-oxohexyl)-1H-purine-2,6(3H,7H)-dione(Lomifylline API). 98%View Details
10226-54-7 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5