L-Sulforaphane
Synonym(s):(R)-1-Isothiocyanato-4-(methylsulfinyl)butane;4-Methylsulfinylbutyl isothiocyanate
- CAS NO.:142825-10-3
- Empirical Formula: C6H11NOS2
- Molecular Weight: 177.29
- MDL number: MFCD09039283
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-08 19:11:55
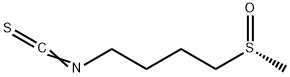
What is L-Sulforaphane?
Description
Sulforaphane is a naturally-occurring phytochemical belonging to the class of isothiocyanates. As the aglycone metabolite of glucosinolate glucoraphanin (sulforaphane glucosinolate), sulforaphane acts as an antioxidant and potent stimulator of endogenous detoxifying enzymes. This agent displays anticarcinogenic properties due to its ability to induce phase II detoxification enzymes, such as glutathione S-transferase and quinone reductase, thereby providing protection against certain carcinogens and toxic, reactive oxygen species. Broccoli sprouts contain large amounts of sulforaphane, which is also found in other cruciferous vegetables including cabbage and kale.
Chemical properties
L-Sulforaphane is a clear Pale Yellow Oil and It is an isothiocyanate derived from the natural compound glucoraphanin, which is abundant in cruciferous vegetables, including broccoli. soluble in methanol, ethanol, DMSO and other organic solvents.
The Uses of L-Sulforaphane
L-sulforaphane is an Antitumor agent and has antioxidant, anti-cancer, anti-ultraviolet, help clear lung bacteria, and prevent gout. It was found to inhibit chemically induced mammary tumor formation in rats. L-Sulforaphane has been reported to prevent NFκB binding, which results in downregulation of apoptosis inhibitors therefore inducing apoptosis. It also displays a capacity to inhibit intracellular, extracellular, and antibiotic-resistant strains of Helicobacter pylori. Additionally, L-Sulforaphane has been found to prevent clonogenicity. This Sulforaphane is sold in the L-enantiomer form.
What are the applications of Application
L-Sulforaphane was used to study Nrf2-mediated signaling in mouse primary preadipocytes and murine macrophage RAW 264.7 cell line.
What are the applications of Application
L-Sulforaphane is an inducer of apoptosis, phase II detoxification enzymes, and xenobiotic enzymes.
Definition
ChEBI: (R)-sulforaphane is a sulforaphane in which the sulfinyl group has R configuration. Naturally occurring compound found in brocolli that acts as a (R)-sulforaphane is a sulforaphane in which the sulfinyl group has R configuration. Naturally occurring compound found in brocolli that acts as a potent inducer of phase II detoxification enzymes. It is an enantiomer of a (S)-sulforaphane.potent inducer of phase II detoxification enzymes. It is an enantiomer of a (S)-sulforaphane.
Biochem/physiol Actions
L-Sulforaphane is a potent, selective inducer of phase II detoxification enzymes with anticarcinogenic properties. Organosulfur compound found in cruciferous vegetables, including broccoli.
Sulforaphane is an anti-cancer, anti-microbial and anti-diabetic compound found in cruciferous vegetables. It induces the production of detoxifying enzymes such as quinone reductase and glutathione S-transferase that cause xenobiotic transformation. Sulforaphane also increases the transcription of tumor suppressor proteins and inhibits histone deacetylases. It modulates inflammatory responses by suppressing the LPS-mediated expression of iNOS, COX-2, IL-1β and TNF-α.
Properties of L-Sulforaphane
| Boiling point: | 368.2±25.0 °C(Predicted) |
| Density | 1.17±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: 40 mg/mL, soluble |
| form | liquid |
| color | slightly yellow |
Safety information for L-Sulforaphane
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for L-Sulforaphane
| InChIKey | SUVMJBTUFCVSAD-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 L-Sulforaphane CAS 142825-10-3View Details
L-Sulforaphane CAS 142825-10-3View Details
142825-10-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
