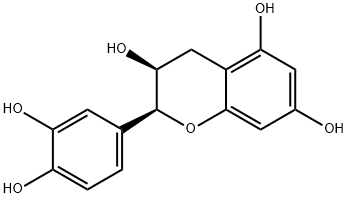
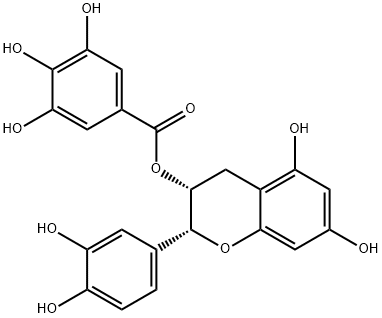
L-Epicatechin
Synonym(s):(−)-cis-3,3′,4′,5,7-Pentahydroxyflavane;(2R,3R)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
- CAS NO.:490-46-0
- Empirical Formula: C15H14O6
- Molecular Weight: 290.27
- MDL number: MFCD00075648
- EINECS: 207-710-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-23 16:19:27
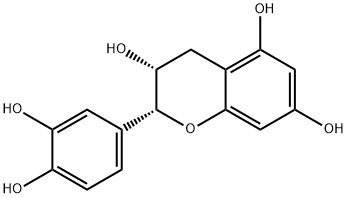
What is L-Epicatechin ?
Description
(−)-Epicatechin is a polyketide synthase-derived polyphenol flavonoid that has been found in T. cacao and has diverse biological activities. It scavenges DPPH radicals in a cell-free assay when used at a concentration of 5 μM. (−)-Epicatechin inhibits COX-1 (IC50 = 3.2 μM). It acts synergistically with epigallocatechin gallate to induce apoptosis in, and reduce the proliferation of, PC-9 lung cancer cells when used at a concentration of 200 μM. (−)-Epicatechin (80 mg/kg) reduces LPS-induced increases in plasma creatinine and urea levels in a rat model of renal inflammation.
Chemical properties
white to light yellow crystal powde
The Uses of L-Epicatechin
Potent antioxidant and antineoplastic agent.
The Uses of L-Epicatechin
Catechin is a polyphenolic flavonoid that has been isolated from a variety of natural sources including tea leaves, grape seeds, and the wood and bark of trees such as acacia and mahogany. Catechin is a more potent antioxidant than ascorbate or α-tocopherol in certain in vitro lipid peroxidation assays. (?)-Epicatechin is a 2R,3R stereoisomer of catechin. Like catechin, (?)-epicatechin is a powerful antioxidant. It inhibits cyclooxygenase 1 (IC50 = 3.2 μM). Also, at 100 μM, (?)-epicatechin induces apoptosis in human adenocarcinoma PC-9 cells and stimulates the inhibition of tumor necrosis factor-α release from BALB-c/3T3 cells treated with epigallocatechin gallate.
The Uses of L-Epicatechin
An antioxidant and natural product from green tea
What are the applications of Application
(?)Epicatechin is a natural product from green tea
Definition
ChEBI: A catechin with (2R,3R)-configuration.
General Description
(?)-Epicatechin (EC) belongs to the group of flavanols and is abundantly present in cacao and cacao products. The chemical structure of EC includes an oxygenated heterocycle with a 4-hydroxyl group linked with two aromatic rings.
Biochem/physiol Actions
This antioxidant is found in chocolate. (-)-Epicatechin is linked with cardiovascular effects. It is considered as a dependable standard due to its availability in cacao seeds and cocoa products. The halogenating activity of myeloperoxidase can be restored by (-)-epicatechin.
References
[1]. chakravarthy bk, gupta s, gambhir ss, et al. pancreatic beta-cell regeneration in rats by (-)-epicatechin. lancet, 1981, 2(8249): 759-760.
[2]. wippel r, rehn m, gorren ac, et al. interference of the polyphenol epicatechin with the biological chemistry of nitric oxide- and peroxynitrite-mediated reactions. biochem pharmacol, 2004, 67(7): 1285-1295.
[3]. brossette t, hundsdörfer c, kröncke kd, et al. direct evidence that (-)-epicatechin increases nitric oxide levels in human endothelial cells. eur j nutr, 2011, 50(7): 595-599.
[4]. okushio k, suzuki m, matsumoto n, et al. identification of (-)-epicatechin metabolites and their metabolic fate in the rat. drug metab dispos, 1999, 27(2): 309-316.
Properties of L-Epicatechin
| Melting point: | 240 °C (dec.)(lit.) |
| Boiling point: | 629.2 °C |
| alpha | -55~-65゜(D/20℃)(c=1,CH3OH) |
| Density | 1.593±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥14.5 mg/mL in DMSO |
| form | neat |
| pka | 9.54±0.10(Predicted) |
| form | Solid |
| color | White to Light yellow to Light orange |
| Water Solubility | Soluble in water or alcohol |
| Merck | 13,1912 |
| BRN | 92760 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 490-46-0(CAS DataBase Reference) |
Safety information for L-Epicatechin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for L-Epicatechin
| InChIKey | PFTAWBLQPZVEMU-UKRRQHHQSA-N |
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 L-Epicatechin 98% CAS 490-46-0View Details
L-Epicatechin 98% CAS 490-46-0View Details
490-46-0 -
 (−)-Epicatechin, ≥90% (HPLC) CAS 490-46-0View Details
(−)-Epicatechin, ≥90% (HPLC) CAS 490-46-0View Details
490-46-0 -
 (-)-Epicatechin CAS 490-46-0View Details
(-)-Epicatechin CAS 490-46-0View Details
490-46-0 -
 (−)-Epicatechin, ≥98% (HPLC) CAS 490-46-0View Details
(−)-Epicatechin, ≥98% (HPLC) CAS 490-46-0View Details
490-46-0 -
 Epicatechin CAS 490-46-0View Details
Epicatechin CAS 490-46-0View Details
490-46-0 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
