kukoamine A
- CAS NO.:75288-96-9
- Empirical Formula: C28H42N4O6
- Molecular Weight: 530.66
- MDL number: MFCD29904538
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:13:54
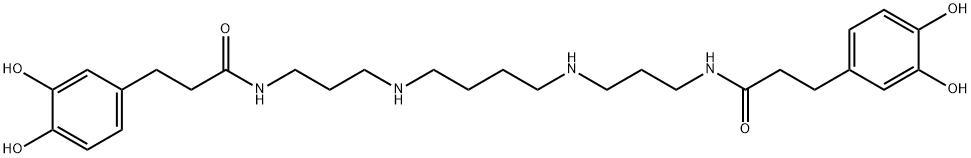
What is kukoamine A?
Description
Kukoamine A is a spermine alkaloid originally isolated from L. chinense that has diverse biological activities, including anticancer, neuroprotective, and anti-inflammatory properties. Kukoamine A (5-20 μg/ml) inhibits colony formation of U251 and WJ1 glioblastoma cells in a concentration-dependent manner. It halts the cell cycle at the G0/G1 phase and induces apoptosis when used at concentrations of 60 and 80 μg/ml. Kukoamine A (20 and 40 μM) induces autophagy and increases cell viability in an SH-SY5Y cell model of MPP-induced injury. It increases the number of dopamine neurons in the substantia nigra and striatum, decreases α-synuclein expression, and improves motor function in an MPTP mouse model of Parkinson’s disease when administered at a dose of 20 mg/kg per day. Kukoamine A (10 and 20 mg/kg) decreases IL-1β, TNF-α, and COX-2 protein levels in the hippocampus and increases hippocampal neurogenesis in a rat model of radiation injury. It also selectively inhibits trypanothione reductase (Ki = 1.8 μM), an enzyme that protects certain parasites from oxidative stress, over human glutathione reductase (Ki = >10 mM).
Occurrence
The root bark of Lychium chinense yields this alkaloid. Kukoamine A possesses hypotensive activity inducing hypotension in rats at a dose of 5 mg/kg when given intravenously.
The Uses of kukoamine A
Kukoamine A is a neuroprotective agent which is used to prevent the loss of dopaminergic neurons in substantia nigra.
Definition
ChEBI: Kukoamine A is an amine.
Properties of kukoamine A
| Boiling point: | 872.1±65.0 °C(Predicted) |
| Density | 1.213±0.06 g/cm3 (20 ºC 760 Torr) |
| storage temp. | Store at -20°C |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: 30 mg/ml; PBS (pH 7.2): 10 mg/ml |
| form | A crystalline solid |
| pka | 9.48±0.10(Predicted) |
| color | Off-white to yellow |
| CAS DataBase Reference | 75288-96-9 |
Safety information for kukoamine A
Computed Descriptors for kukoamine A
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Kukoamine A CAS 75288-96-9View Details
Kukoamine A CAS 75288-96-9View Details
75288-96-9 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
