IRON (II) FLUORIDE
Synonym(s):Ferrous fluoride;Iron difluoride
- CAS NO.:7789-28-8
- Empirical Formula: F2Fe
- Molecular Weight: 93.84
- MDL number: MFCD00016093
- EINECS: 232-155-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:58
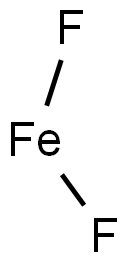
What is IRON (II) FLUORIDE?
Chemical properties
brown fine crystalline powder
Chemical properties
Iron(II) fluoride is prepared by passing hydrogen fluoride over iron at red heat or over iron(II) chloride at a somewhat lower temperature. It is sparingly soluble in water with some hydrolysis, and is insoluble in alcohol, ether and benzene. The boiling point is about 1100°C and the melting point only a little below this temperature.
Physical properties
White tetragonal crystal; density 4.09g/cm3; melts at 1100°C; slightly soluble in water; insoluble in ethanol and ether; dissolves in dilute hydrofluoric acid. Tetrahydrate crystals are hexagonal shape; density 2.20g/cm3; decomposes at 100°C.
.
The Uses of IRON (II) FLUORIDE
Iron(II) fluoride is used as a catalyst in organic fluorination reactions. Other applications are in ceramics; and in the preparation of fluoride salts of other metals.
The Uses of IRON (II) FLUORIDE
Iron(II) fluoride is used for proteomics research applications. It is also a chemical intermediate.
Structure and conformation
Iron(II) fluoride crystallizes in the rutile structure, the iron atom being surrounded tetragonally91 by 4F- at 2·12 ? and 2F- at 1·99 ?. Iron(II) fluoride is reduced to iron by hydrogen at red heat, but is stable to hydrogen at 400°—and can be prepared by reduction of iron(III) fluoride with hydrogen at this temperature. It reacts violently when heated with metals such as sodium and aluminium, but undergoes no visible reaction with bromine, iodine or sulphur. The ammoniates FeF2.5NH3,H20, FeF2.NH3.H20 and FeF2.0·5NH3.H2O are obtained in the reactions of gaseous ammonia with the tetrahydrate at various temperatures.
Properties of IRON (II) FLUORIDE
| Melting point: | >1000°C |
| Density | 4,09 g/cm3 |
| solubility | slightly soluble in H2O; soluble in dilute HF; insoluble in EtOH, ethyl ether |
| form | Powder |
| Specific Gravity | 4.09 |
| color | White |
| Water Solubility | Insoluble in water. |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,4045 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7789-28-8(CAS DataBase Reference) |
| EPA Substance Registry System | Iron fluoride (FeF2) (7789-28-8) |
Safety information for IRON (II) FLUORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for IRON (II) FLUORIDE
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
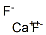
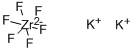
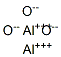
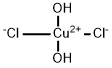
You may like
-
 Iron(II) fluoride, Anhydrous CAS 7789-28-8View Details
Iron(II) fluoride, Anhydrous CAS 7789-28-8View Details
7789-28-8 -
 Iron(II) fluoride, Anhydrous CAS 7789-28-8View Details
Iron(II) fluoride, Anhydrous CAS 7789-28-8View Details
7789-28-8 -
 Ferrous fluoride, 98% CAS 7789-28-8View Details
Ferrous fluoride, 98% CAS 7789-28-8View Details
7789-28-8 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
