Indole-4-carboxaldehyde
Synonym(s):4-Formylindole;NSC 337264
- CAS NO.:1074-86-8
- Empirical Formula: C9H7NO
- Molecular Weight: 145.16
- MDL number: MFCD01632221
- EINECS: 625-162-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:17
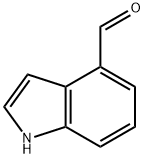
What is Indole-4-carboxaldehyde?
Chemical properties
White to dark brown solid
The Uses of Indole-4-carboxaldehyde
Indole-4-carboxaldehyde is usually used as reactant in Biginelli reaction,preparation of antitumor agents,intramolecular Friedel-Crafts acylation,preparation of inhibitors of cell division in E. Coli,synthesis of Hantzsch pyridine-containing Schiff bases.
The Uses of Indole-4-carboxaldehyde
4-Indolecarbaldehyde is a synthetic intermediate used for pharmaceutical synthesis. Also used as reactant in Biginelli reaction, synthesis of aurora kinase A inhibitors, preparation of antitumor agents, intermolecular Friedel-Crafts acylation, preparation of inhibitors of cell division in E. coli and synthesis of Hantzsch pyridine-containing Schiff bases.
What are the applications of Application
4-formyl Indole is a synthetic intermediate
Definition
ChEBI: Indole-4-carbaldehyde is a heteroarenecarbaldehyde that is indole in which the hydrogen at position 4 has been replaced by a formyl group. It has a role as an algal metabolite. It is a member of indoles and a heteroarenecarbaldehyde.
Synthesis Reference(s)
The Journal of Organic Chemistry, 46, p. 1752, 1981 DOI: 10.1021/jo00321a053
Tetrahedron, 39, p. 3695, 1983 DOI: 10.1016/S0040-4020(01)88608-7
General Description
Indole-4-carboxaldehyde participates in the synthesis of arcyriacyanin A. Synthesis of 4-indole-4-carboxaldehyde has been reported. Intramolecular Friedel-Crafts (FC) acylation of indole-4-carboxaldehyde has been reported.
Synthesis
Tetrapropylammonium perruthenate (0.3 g, 0.85 mmol) was added in portions to a mixture of alcohol product from 1H-Indole-4-Methanol (2.5 g, 17 mmol), N-methylmorpholine N-oxide (3.0 g, 25 mmol) and 4 A molecular sieves (3.0 g) in anhydrous methylene chloride (30 mL) at room temperature. The mixture was stirred at room temperature under nitrogen for 1 h and then filtered. The filtrate was concentrated in vacuo, and the residue was purified by chromatography (SiO2, CH2Cl2) to provide Indole-4-carboxaldehyde as a white powder (2.0 g, 80percent): 1H NMR (300 MHz, CDCl3) δ10.2 (s, 1H), 8.52 (br s, 1H), 7.64-7.69 (m, 2H), 7.31-7.44 (m, 3H), CI MS m/z=146 [C9H7NO+H]+.
Properties of Indole-4-carboxaldehyde
| Melting point: | 139-143 °C(lit.) |
| Boiling point: | 339.1±15.0 °C(Predicted) |
| Density | 1.278±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMF: 10 mg/ml; DMSO: 3 mg/ml; Ethanol: 10 mg/ml; Ethanol:PBS(pH 7.2) (1:1): 0.5 mg/ml |
| pka | 15.84±0.30(Predicted) |
| form | powder to crystal |
| color | Light yellow to Brown |
| Water Solubility | Soluble in ethanol and acetone. Insoluble in water. |
| Sensitive | Air Sensitive |
| InChI | InChI=1S/C9H7NO/c11-6-7-2-1-3-9-8(7)4-5-10-9/h1-6,10H |
| CAS DataBase Reference | 1074-86-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-indole-4-carboxaldehyde(1074-86-8) |
Safety information for Indole-4-carboxaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Indole-4-carboxaldehyde
| InChIKey | JFDDFGLNZWNJTK-UHFFFAOYSA-N |
| SMILES | N1C2=C(C(C=O)=CC=C2)C=C1 |
New Products
Cyclopropane-1,1-dicarboxylic acid Cyclopentane-1,2-dione 2-Bromo-5-iodopyridine 4-isothiocyanato-2-(trifluoroMethyl)benzonitrile Amino-4-methoxyben-zeneacetic acid 3-Aminophenylacetic acid 1-AMino-cyclobutaneMethanol HCl (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Cefuroxime EP Impurity-A N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 4-Bromo Benzylcyanide 5-azidovalericacid 4-bromo-1H-pyrazole-3-carboxylic acid Isopropyl amine Hydrochloride tert-butyl 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-vinyl-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)piperazine-1-carboxylateRelated products of tetrahydrofuran
You may like
-
 Indole-4-carboxaldehyde CAS 1074-86-8View Details
Indole-4-carboxaldehyde CAS 1074-86-8View Details
1074-86-8 -
 Indole-4-carboxaldehyde 98% (HPLC) CAS 1074-86-8View Details
Indole-4-carboxaldehyde 98% (HPLC) CAS 1074-86-8View Details
1074-86-8 -
 4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
6939-69-1 -
 1-(cyanomethyl)-1-cyclopentanecarbonitrile 98+View Details
1-(cyanomethyl)-1-cyclopentanecarbonitrile 98+View Details
1539-03-3 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8
