Indigo
Synonym(s):Indigo blue;Indigotin
- CAS NO.:482-89-3
- Empirical Formula: C16H10N2O2
- Molecular Weight: 262.26
- MDL number: MFCD00005722
- EINECS: 207-586-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-24 18:36:04
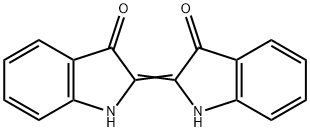
What is Indigo?
Description
Indigo, known chemically as indigotin, is a common blue dye that has been highly valued
throughout history and has played a major role in trade and commerce since ancient times.
The term indigo is often used to describe many blue dyes produced from a number of plants.
For example, woad, a blue dye obtained from the plant Isatis tinctoria, was used throughout
the Mediterannean and Europe and is often identified as indigo. True indigo comes from
the leguminous plant of the genus Indigofera.
The Indigofera genus includes several hundred
species, and indigo has been obtained from a number of these, but the dominant species for
the dye are Indigofera tinctoria grown mainly in India and tropical Asia and Indigofera suff ructiosa
from the tropical Americas. The name indigo comes from the Greek indikon and Latin
indicum meaning “dye from India.” There is evidence that indigo was used several thou sand
years b.c.e. Persian rugs containing indigo color exist from several thousand years b.c.e. Textile
artifacts from Egyptian tombs provide evidence of indigo’s use by royalty from as far back as
2500 b.c.e. The writings of Herodotus from approximately 450 b.c.e. mention indigo’s use in
the Mediterranean area.
Description
Indigo, sometimes called indigo dye, is probably the oldest dye or pigment used by humans. It was originally obtained from plants in the?Indigofera?and?Isatisgenera. In 1878, A. von Baeyer synthesized it from isatin; 12 years later, K. Heumann developed a commercial process; and by the end of the 19th century, most indigo dye was synthetic. Indigo is insoluble in water, so?dyers treat yarn with alkaline solutions?of the yellow reduced form leucoindigo, which oxidizes to indigo when the yarn is exposed to air.
Chemical properties
dark violet powder
Occurrence
Indigo is a perennial shrub found in several regions of the world.
History
Indigotin. The blue dye of the ancient world was derived from indigo and woad. Which plant is the oldest is a matter of conjecture. That indigo was known at least four thousand years ago is evident from ancient Sanskrit writings. Cloth dyed with indigotin (CI Natural Blue; CI 75780) has been found in Egyptian tombs and in the graves of the Incas in South America. Indigo belongs to the legume family. The two most important species are Indigo tinctoria and I. suffruticosa, found in India and the Americas, respectively. The leaves of the indigo plant do not contain the dye as such, but in the form of its precursor, a glycoside known as indican.
The Uses of Indigo
As textile dye. In sutures.
The Uses of Indigo
Indigo is a chemical compound used as a dye in industrial clothing and textile processes. Also used in the synthesis of organic semiconductors. Dyes and metabolites.
The Uses of Indigo
In recent years researchers have used genetic engineering using Escherichia coli to convert tryptophan into indigo. The desire for natural organic products has also revived traditional production methods of indigo on a small scale. Indigo's dominant use is as a textile dye, but indigo-related compounds have limited use as indicators and in food coloring.the Food and Drug Administration's FD&C Blue #2 contains indigotine (also known as indigo carmine), which is a sulfonated sodium salt of indigo.
What are the applications of Application
Indigo is an indole stain
Production Methods
K. Heumann treated N-phenylglycine with alkali and obtained indoxyl (keto form), which on aerial oxidation converted to indigotin. Later, a variation of the original Heumann process was made: aniline, formaldehyde, and hydrogen cyanide react to form phenylglycinonitrile, which is hydrolyzed to phenylglycine. This is the most widely used process for manufacturing indigotin. The greatest improvement in the manufacture of indigotin came when sodamide was used with alkali in the conversion of phenylglycine to indoxyl. Although there is still demand for indigotin for dyeing blue jeans, it has lost a good part of the market to other blue dyes with better dyeing properties.
Definition
A double indole derivative.
Definition
indigo: A blue vat dye, C16H10N2O2.It occurs as the glucoside indican inthe leaves of plants of the genus Indigofera,from which it was formerlyextracted. It is now made synthetically.
Production Methods
The first synthesis of indigo is attributed to Adolf von Baeyer (1835–1917), who began hisquest to synthesize indigo in 1865 but was not able to produce indigo until 1878. The syntheticproduction of indigo was first described by Baeyer and Viggo Drewson in 1882; Baeyeralso identified the structure of indigo in 1882.the Baeyer-Drewson synthesis of indigo startedwith 2-nitrobenzaldehyde and acetone proceeding through a series of steps in alkali solution.Baeyer’s work was not commercially viable, and it was not until 1897 that BASF (BadischeAnalin und Soda Fabrik) started to produce indigo commercially using a process developedby Karl von Heumann (1851–1894) that started with naphthalene. The synthetic productionof indigo spelled the end of traditional methods of indigo production. By the second decadeof the 20th century, nearly all indigo was produced synthetically.
Biological Functions
Indigo naturalis has a variety of pharmacological activities, such as anti-inflammatory, antioxidant, antibacterial, antiviral, immunomodulatory and so on. It has very good clinical effect on psoriasis, leukemia and ulcerative colitis.
General Description
Dark blue powder with coppery luster. Occurs in isomeric forms (cis and trans). In solid state Indigo is in the trans form.
Air & Water Reactions
Insoluble in water.
Health Hazard
ACUTE/CHRONIC HAZARDS: Indigo may cause irritation of the skin and mucous membranes.
Fire Hazard
Flash point data for Indigo are not available but Indigo is probably combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx.
Properties and Applications
|
TEST ITEMS |
SPECIFICATION |
|
APPEARANCE |
BLUE POWDER |
|
SHADE |
GREENISH |
|
HEAT RESISTANCE |
180 °C min |
|
LIGHT FASTNESS |
7 |
|
ACID RESISTANCE |
3 |
|
ALKALI RESISTANCE |
4 |
|
FASTNESS TO BLEEDING |
4 |
|
OIL ABSORPTION |
40-50% |
|
SPECIFIC SURFACE |
27 m 2 /g |
|
DENSITY |
1.60 g/cm 3 |
|
RESIDUE ON 80 MESH |
5.0% max |
|
WATER SOLUBLE |
1.0% max |
|
VOLATITE 105 °C |
1.0% max |
|
TINTING STRENGTH |
100-105 % |
Purification Methods
First reduce indigo in alkaline solution with sodium hydrosulfite, and filter. The filtrate is then oxidised by air, and the resulting precipitate is filtered off, dried at 65-70o, ground to a fine powder, and extracted with CHCl3 in a Soxhlet extractor. Evaporation of the CHCl3 extract gives the purified dye. [Brode et al. J Am Chem Soc 76 1034 1954; spectral characteristics are listed, Beilstein 24 II 233, 24 III/IV 1791.]
Properties of Indigo
| Melting point: | >300 °C(lit.) |
| Boiling point: | 405.51°C (rough estimate) |
| Density | 1.01 g/mL at 20 °C |
| vapor pressure | 0Pa at 100℃ |
| refractive index | 1.5800 (estimate) |
| Flash point: | >220℃ |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly, Heated, Sonicated), DMF (Slightly) |
| Colour Index | 73000 |
| form | Powder |
| pka | -3.83±0.20(Predicted) |
| color | Dark blue to violet |
| Water Solubility | <0.1 g/100 mL |
| Merck | 14,4943 |
| BRN | 88275 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 482-89-3(CAS DataBase Reference) |
| NIST Chemistry Reference | c.i. Vat blue 1(482-89-3) |
| EPA Substance Registry System | C.I. Vat Blue 1 (482-89-3) |
Safety information for Indigo
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Indigo
| InChIKey | COHYTHOBJLSHDF-BUHFOSPRSA-N |
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran



You may like
-
 482-89-3 Pigment blue 66 98%View Details
482-89-3 Pigment blue 66 98%View Details
482-89-3 -
 482-89-3 98%View Details
482-89-3 98%View Details
482-89-3 -
 Indigo (synthetic) CAS 482-89-3View Details
Indigo (synthetic) CAS 482-89-3View Details
482-89-3 -
 Indigo 97% (N) CAS 482-89-3View Details
Indigo 97% (N) CAS 482-89-3View Details
482-89-3 -
 Indigo CAS 482-89-3View Details
Indigo CAS 482-89-3View Details
482-89-3 -
 Chevron Indigo Cotton Printed FabricView Details
Chevron Indigo Cotton Printed FabricView Details
482-89-3 -
 Embroidered Indigo 322640 Non Stretch Woven Jacquard Denim Fabric, For Shirts, Packaging Type: RollView Details
Embroidered Indigo 322640 Non Stretch Woven Jacquard Denim Fabric, For Shirts, Packaging Type: RollView Details
482-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8
