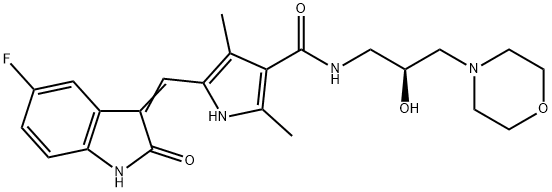
Glecaprevir
- CAS NO.:1365970-03-1
- Empirical Formula: C38H46F4N6O9S
- Molecular Weight: 838.87
- MDL number: MFCD30533436
- Update Date: 2026-01-20 10:51:09
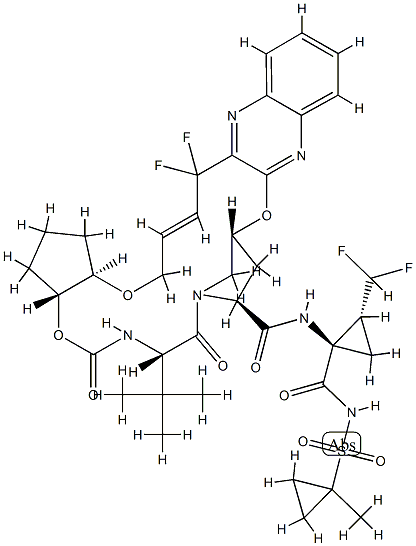
What is Glecaprevir?
Absorption
In healthy subjects, the time it takes to reach the peak plasma concentration (Tmax) is approximately 5 hours. The mean peak plasma concentration (Cmax) is 597ng/mL in non-cirrhotic HCV-infected subjects. Relative to fasting conditions, the consumption of meals increases the absorption of glecaprevir by 83-163% .
Toxicity
Glecaprevir is not shown to be genotoxic according to in vitro or in vivo studies. It also shows to have no effect on mating, female or male fertility, or early embryonic development in rodent studies. Carcinogenicity studies with glecaprevir have not been conducted .
The Uses of Glecaprevir
Glecaprevir (INN) is a hepatitis C virus (HCV) nonstructural (NS) protein 3/4A protease inhibitor that was identified jointly by AbbVie and Enanta Pharmaceuticals. It is being developed as a treatment of chronic hepatitis C infection in co-formulation with an HCV NS5A inhibitor pibrentasvir. Together they demonstrated potent antiviral activity against major HCV genotypes and high barriers to resistance in vitro.
The Uses of Glecaprevir
Glecaprevir is an antiviral drug used in the treatment of patients with hepatitis C virus (HCV) genotype 1-6, of special interest for the treatment of patients with chronic kidney disease also suffering from HCV.
Background
Glecaprevir is a direct acting antiviral agent and Hepatitis C virus (HCV) NS3/4A protease inhibitor that targets the the viral RNA replication. In combination with Pibrentasvir, glecaprevir is a useful therapy for patients who experienced therapeutic failure from other NS3/4A protease inhibitors. It demonstrates a high genetic barrier against resistance mutations of the virus. In cell cultures, the emergence of amino acid substitutions at NS3 resistance-associated positions A156 or D/Q168 in HCV genotype 1a, 2a or 3a replicons led to reduced susceptibility to glecaprevir . The combinations of amino acid substitutions at NS3 position Y65H and D/Q168 also results in greater reductions in glecaprevir susceptibility, and NS3 Q80R in genotype 3a patients also leads to glecaprevir resistance .
Glecaprevir is available as an oral combination therapy with Pibrentasvir under the brand name Mavyret. This fixed-dose combination therapy was FDA-approved in August 2017 to treat adults with chronic hepatitis C virus (HCV) genotypes 1-6 without cirrhosis (liver disease) or with mild cirrhosis, including patients with moderate to severe kidney disease and those who are on dialysis . Mavyret is also indicated for HCV genotype 1-infected patients who have been previously treated with regimens either containing an NS5A inhibitor or an NS3/4A protease inhibitor, but not both . Hepatitis C viral infection often leads to decreased liver function and subsequent liver failure, causing a significantly negative impact on the patients' quality of life. The ultimate goal of the combination treatment is to achieve sustained virologic response (SVR) and cure the patients from the infection. In clinical trials, this combination therapy achieved SVR12 rate, or undetectable Hepatitis C for twelve or more weeks after the end of treatment, of ≥93% across genotypes 1a, 2a, 3a, 4, 5 and 6 .
Indications
Indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A). MAVYRET is also indicated for the treatment of adult patients with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both .
Pharmacokinetics
In a biochemical assay studying clinical isolates of HCV genotypes 1a, 1b, 2a, 2b, 3a, 4a, 5a, and 6a, glecaprevir displayed IC50 values ranging from 3.5 to 11.3 nM that resulted in inhibition of the proteolytic activity of recombinant NS3/4A enzymes. In HCV replicon assays, glecaprevir had median EC50 values of 0.08-4.6 nM against laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4d, 5a, and 6a . In a QT study, glecaprevir is not shown to prolong the QTc interval.
Side Effects
The most common adverse effects, observed in at least 10% of phase 3 trial participants, were headache and fatigue.
Metabolism
Glecaprevir undergoes limited secondary metabolism in vitro, predominantly by CYP3A .
Properties of Glecaprevir
| Melting point: | >186°C (dec.) |
| Density | 1.46±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 4.46±0.40(Predicted) |
| form | Solid |
| color | White to Off-White |
Safety information for Glecaprevir
Computed Descriptors for Glecaprevir
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
