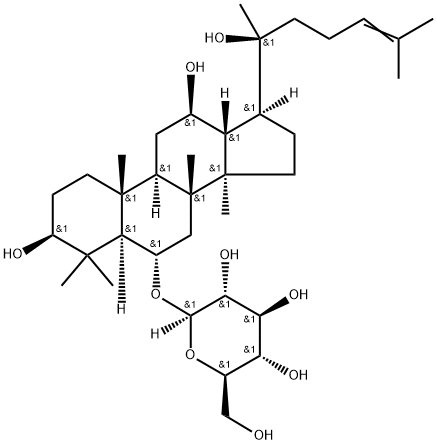
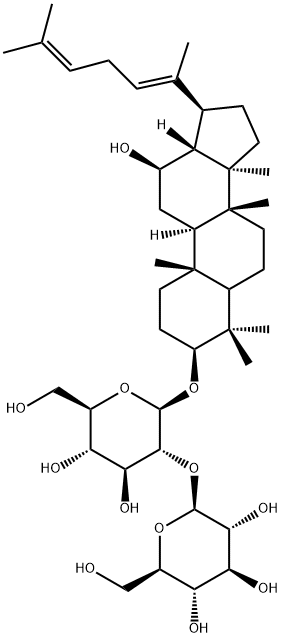
Ginsenoside Rh2
Synonym(s):(3β,12β)-12,20-Dihydroxydammar-24-en-3-yl-β-D -glucopyranoside;20(S)-Ginsenoside-Rh2
- CAS NO.:78214-33-2
- Empirical Formula: C36H62O8
- Molecular Weight: 622.88
- MDL number: MFCD00800712
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
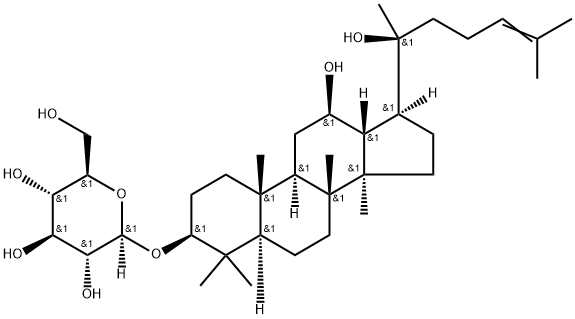
What is Ginsenoside Rh2?
Description
Ginsenoside Rh2 is a steroid glycoside found in plants of the genus Panax that has diverse biological activities. It inhibits release of β-hexosaminidase from RBL-2H3 cells (IC50 = 100 μM) and inhibits the IgE-dependent passive cutaneous anaphylaxis reaction in mice at a dose of 25 mg/kg. It inhibits growth of HRA ovarian and BxPC-3 pancreatic cancer cells in a dose-dependent manner. In vivo, ginsenoside Rh2 decreases immobility time in the forced swim test in a mouse model of colorectal carcinoma. Ginsenoside Rh2 also reduces infarct volume in a rat model of ischemia-reperfusion-induced brain injury.
Chemical properties
White solid
The Uses of Ginsenoside Rh2
20(S)-Ginsenoside Rh2 is a bioactive metabolite of the ginsenoside component of Panax ginseng that has been shown to exhibit antibacterial effects in vitro.
What are the applications of Application
20(S)- Ginsenoside Rh2 is a plant glycoside with anti-cancer proliferation and chemopreventive effects
Definition
ChEBI: A ginsenoside found in Panax species that is dammarane which is substituted by hydroxy groups at the 3beta, 12beta and 20 pro-S positions, in which the hydroxy group at position 3 has been converted to the corresponding beta-D-glucopyranoside, and in which a double bond has been introduced at the 24-25 position.
Properties of Ginsenoside Rh2
| Melting point: | 139-144 °C |
| Boiling point: | 726.4±60.0 °C(Predicted) |
| Density | 1.19±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMF: 10 mg/ml; DMSO: 10 mg/ml; DMSO:PBS (pH 7.2) (1:1): 0.5 mg/ml |
| form | neat |
| pka | 12.91±0.70(Predicted) |
| form | Solid |
| color | White |
| CAS DataBase Reference | 78214-33-2(CAS DataBase Reference) |
Safety information for Ginsenoside Rh2
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ginsenoside Rh2
| InChIKey | CKUVNOCSBYYHIS-IRFFNABBSA-N |
New Products
Cyclopropane-1,1-dicarboxylic acid Cyclopentane-1,2-dione 2-Bromo-5-iodopyridine 4-isothiocyanato-2-(trifluoroMethyl)benzonitrile Amino-4-methoxyben-zeneacetic acid 3-Aminophenylacetic acid 1-AMino-cyclobutaneMethanol HCl (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Cefuroxime EP Impurity-A N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 4-Bromo Benzylcyanide 5-azidovalericacid 4-bromo-1H-pyrazole-3-carboxylic acid Isopropyl amine Hydrochloride tert-butyl 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-vinyl-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)piperazine-1-carboxylateRelated products of tetrahydrofuran
You may like
-
 Ginsenoside Rh2 CAS 78214-33-2View Details
Ginsenoside Rh2 CAS 78214-33-2View Details
78214-33-2 -
 4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
4-cyano-2,2-dimethylbutanoic acid 6939-69-1 98+View Details
6939-69-1 -
 1-(cyanomethyl)-1-cyclopentanecarbonitrile 98+View Details
1-(cyanomethyl)-1-cyclopentanecarbonitrile 98+View Details
1539-03-3 -
 tert-butyl(R)-4-(methyl(1-(pyrrolidin-3-yl)cyclopropyl)amino)piperidine-1-carboxylate 922718-06-7 98+View Details
tert-butyl(R)-4-(methyl(1-(pyrrolidin-3-yl)cyclopropyl)amino)piperidine-1-carboxylate 922718-06-7 98+View Details
922718-06-7 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8 -
 39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 98+View Details
39773-47-2 -
 1392213-15-8 98+View Details
1392213-15-8 98+View Details
1392213-15-8
