78214-33-2
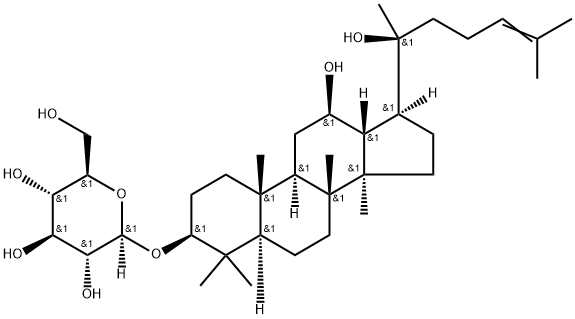
Product Name:
Ginsenoside Rh2
Formula:
C36H62O8
Synonyms:
(3β,12β)-12,20-Dihydroxydammar-24-en-3-yl-β-D -glucopyranoside;20(S)-Ginsenoside-Rh2
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 622.9 g/mol |
|---|---|
| XLogP3 | 5.6 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 7 |
| Exact Mass | 622.44446893 g/mol |
| Monoisotopic Mass | 622.44446893 g/mol |
| Topological Polar Surface Area | 140 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1070 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 15 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
(20S)-ginsenoside Rh2 is a ginsenoside found in Panax species that is dammarane which is substituted by hydroxy groups at the 3beta, 12beta and 20 pro-S positions, in which the hydroxy group at position 3 has been converted to the corresponding beta-D-glucopyranoside, and in which a double bond has been introduced at the 24-25 position. It has a role as a plant metabolite, an antineoplastic agent, an apoptosis inducer, a cardioprotective agent, a bone density conservation agent and a hepatoprotective agent. It is a beta-D-glucoside, a 12beta-hydroxy steroid, a ginsenoside, a tetracyclic triterpenoid and a 20-hydroxy steroid. It derives from a hydride of a dammarane.
