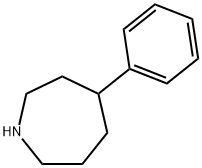
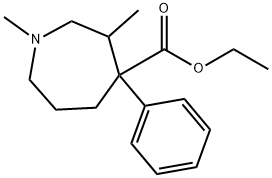
ethoheptazine
- CAS NO.:77-15-6
- Empirical Formula: C16H23NO2
- Molecular Weight: 261.363
- MDL number: MFCD00864347
- EINECS: 201-007-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:42
What is ethoheptazine ?
Chemical properties
Liquid.
Originator
Zactane,Wyeth,US,1957
The Uses of ethoheptazine
Medicine (analgesic).
Background
Ethoheptazine is marketed under the name Zactane. It is a phenazepine based opioid analgesic. It was invented in the 1950s and is related to other drugs such as proheptazine. Ethoheptazine is no longer marketed in the United States.
Definition
ChEBI: Ethoheptazine is a member of azepanes.
Manufacturing Process
As a starting material, phenylacetonitrile was reacted with N-(2-
chloroethyl)dimethylamine. This then underwent the following reaction
sequence.
Preparation of 1-Dimethylamino-3-Cyano-3-Phenyl-6-Bromohexane: 65.8
grams (0.35 mol) of 2-phenyl-4-dimethylaminobutyronitrile in 350 cc of
absolute ether was dripped into a stirred suspension of 17.5 grams (0.45 mol)
of sodamide in 350 cc of absolute ether during 1 hour, keeping the reaction
mixture under a dry nitrogen atmosphere. The mixture was stirred an
additional hour at room temperature and then 1 hour at reflux temperature.
The mixture was diluted with 250 cc of absolute ether, cooled in an ice bath,
then, while stirring, a solution of 74.7 grams (0.37 mol) of trimethylene
bromide in 250 cc of absolute ether added at once. The yellow suspension
continued to be stirred at ice-bath temperature for 1 hour, then at room
temperature for 1 hour, and finally at reflux temperature for 3 hours. The
mixture was cooled and the sodium bromide, which had precipitated in
quantitative yield, was filtered off and washed with ether. The light yellow
ethereal filtrate contained the product. This compound could be stored for
some time in a hydrocarbon solvent, e.g., n-heptane, at +5°C.
Preparation of 4-Phenyl-4-Cyano-N-Methyl Azacycloheptane Methobromide: A
0.1 M nitrobenzene solution of 1-dimethylamino-3-cyano-3-phenyl-6-
bromohexane was kept at 100°C for 1 hour whereby the quaternary salt
precipitated out; MP 246° to 247°C.
Preparation of 4-Phenyl-4-Cyano-N-Methyl Azacycloheptane: 6.2 grams (0.02
mol) of the methobromide quaternary salt was suspended in 150 cc of
tetralin. While vigorously stirring, the mixture was heated to its reflux
temperature, whereupon the solid began to disintegrate and go into solution.
The stirring and refluxing was continued 1 hour, then the mixture cooled,
water added, and the layers separated. The tetralin solution was extracted
with 3 M aqueous hydrochloric acid, the acid extract washed with ether, then
made alkaline with aqueous sodium hydroxide and extracted with ether. The
ether extracts were dried, filtered, and the solvent distilled off. Vacuum
distillation of the liquid residue gave the tertiary amine, BP 119° to
121°C/0.25 mm.
Preparation of 4-Phenyl-4-Carbethoxy-N-Methyl Azacycloheptane: A solution of
8.4 grams (0.04 mol) of the cyclic aminonitrile in 10.6 grams concentrated
sulfuric acid and 2.6 grams water was kept at 110° to 120°C (bathtemperature) for 3 hours. Then, while repeatedly adding absolute ethanol,
95% aqueous ethanol was slowly distilled off during 16 hours. The reaction
mixture was concentrated to 50 cc, cooled, poured into 200 cc of a cold
saturated aqueous solution of sodium carbonate and extracted with ether. The
ether extract after drying and filtering yielded, by distillation, the aminoester,
BP 122° to 124°C/0.3 mm.
Therapeutic Function
Analgesic
Metabolism
Not Available
Properties of ethoheptazine
| Melting point: | 25°C |
| Boiling point: | bp1 133-134°; bp0.5 127-129°; bp0.3 128-130° |
| Density | d426 1.038 |
| refractive index | nD26 1.5210; nD28 1.5220 |
| pka | pKa 8.45 (Uncertain) |
Safety information for ethoheptazine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ethoheptazine
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1