ENNIATIN FROM MICROBIAL SOURCE
- CAS NO.:11113-62-5
- Empirical Formula: C36H63N3O9.C35H61N3O9.C34H59N3O9.C33H57N3O9
- Molecular Weight: 2643.46
- MDL number: MFCD00214209
- Update Date: 2026-01-05 15:21:26
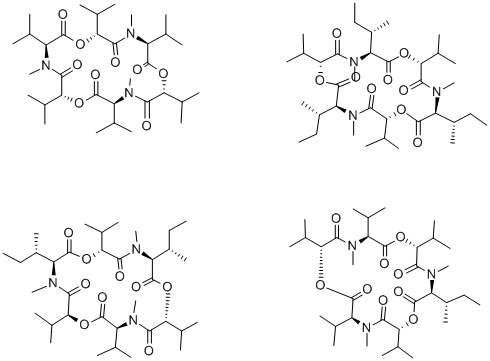
What is ENNIATIN FROM MICROBIAL SOURCE?
The Uses of ENNIATIN FROM MICROBIAL SOURCE
Enniatins are a complex of depsipeptides produced by several Fusarium species. Typically, the complex contains 4 major components: A, A1, B and B1 together with minor amounts of enniatins C, D, E and F. The enniatins act as ionophores. Recently their effects on acyl-CoA cholesterol transferase, as nematocides and the selectivity of their antitumour action have received more focus.
The Uses of ENNIATIN FROM MICROBIAL SOURCE
Enniatin complex is a complex mixture of Fusarium produced depsipeptide ionophores.This compound can be used to control contamination in fermentation of gaseous substrates.
What are the applications of Application
Enniatin complex is a complex mixture of Fusarium produced depsipeptide ionophores
Biological Activity
enniatins, analogues of beauvericin, is a regular cyclic hexadepsipeptide isolated from fusarium species of fungis, also they have been isolated from other genera, such as verticillium and halosarpheia [1]. they have been involved in many biological activities, such as antiinsectan, antifungal, antibiotic and cytotoxic [1].
in vitro
fusafungine is a mixture of several enniatins with bacteriostatic activity against most micro-organisms responsible for infections and superinfections of the respiratory tract. fusafungine has been developed for treatment of upper respiratory tract infections by oral and/or nasal inhalation. fusafungine in low concentrations down-regulated the expression of intercellular adhesion molecule-1 (icam-1) by activated macrophages, inhibited the production of the proinflammatory cytokines il-1, tnfα and il-6 and inhibited the release of oxygen free radicals by inflammatory macrophages without altering their phagocytic activity. fusafungine also inhibited t-cell activation and proliferation, and the synthesis of ifn-γ by activated t cells [2]. exposure 8 h to enniatins at nanomolar concentrations significantly stimulated cell proliferation. at low micromolar concentrations, enniatins exihibited profound apoptosis-inducing effects against various human cancer cell types. in the fluorescence-activated cell sorting analysis, enniatins induced cell cycle arrest in the g0/g1 phase. in human cancer cells, elevated enn concentrations induced profound p53-dependent cytostatic and p53-independent cytotoxic activities [3]. enniatin easily incorporated into the cell membrane in which it formed cation-selective pores [4].
Purification Methods
It is a cyclic peptidic ester antibiotic which is recrystallised from EtOH/water but is deactivated in alkaline solution. [Ovchinnikov & Ivanov in The Proteins (Neurath and Hill eds) Academic Press, NY, Vol V pp. 365 and 516 1982, Tomeda et al. J Antibiot 45 1626 1992.]
References
[1] sy-cordero a a, pearce c j, oberlies n h. revisiting the enniatins: a review of their isolation, biosynthesis, structure determination and biological activities[j]. the journal of antibiotics, 2012, 65(11): 541-549.
[2] german-fattal m. fusafungine, an antimicrobial with anti-inflammatory properties in respiratory tract infections[j]. clinical drug investigation, 2001, 21(9): 653-670.
[3] dornetshuber r, heffeter p, kamyar m r, et al. enniatin exerts p53-dependent cytostatic and p53-independent cytotoxic activities against human cancer cells[j]. chemical research in toxicology, 2007, 20(3): 465-473.
[4] kamyar m, rawnduzi p, studenik c r, et al. investigation of the electrophysiological properties of enniatins[j]. archives of biochemistry and biophysics, 2004, 429(2): 215-223.
Properties of ENNIATIN FROM MICROBIAL SOURCE
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO |
| form | powder |
| color | white |
Safety information for ENNIATIN FROM MICROBIAL SOURCE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. |
Computed Descriptors for ENNIATIN FROM MICROBIAL SOURCE
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
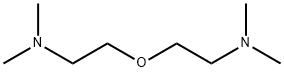

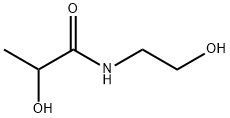
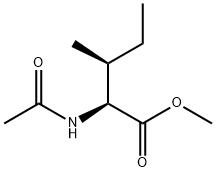
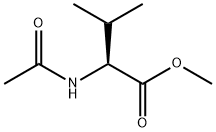
You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
